Herpesviridae: Viral Cycle, Capsid Transport, and Cancer Treatment
Introduction
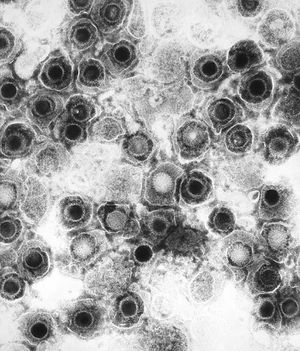
By Michael Gallaher
Herpes is a family of DNA viruses called Herpesviridae. The herpes family of viruses consists of three families of viruses; Alphaherpesviridae, Betaherpesviridae, and Gammaherpesviridae. Among these families are many commonly known viruses that are causative agents of many diseases, such as HSV-1 and HSV-2 for cold sores and genital warts, varicella zoster for chicken pox and shingles, and the Eppstein-Barr virus for mononucleosis. Herpes virsues also tend to have latent, recurring infections in the infected organisms, where the virus remains in some part of the infected organism (Gupta, 2007). Herpes family viruses are incredibly common, with at least 90% of people within the United States having been infected with at least one form of Herpesviridae (CDC, 2006).
Of these viral families, the animal herpes viruses that cause disease are of the Alphaherpesviridae family. The most well known examples of herpes are the herpes simplex viruses, HSV-1 and HSV-2. HSV-1 is the causative pathogen of most oral cold sores, while HSV-2 is the causative agent of genital warts. It is thought that more than 50% of adults in the United States have HSV-1, and that nearly 20% have HSV-2 (CDC, 2010). The widespread infection of the populace makes herpes a good target for medical research. Another promising field of research that has developed more recently is the possibility of using a re-engineered HSV as a treatment vector for other diseases, such as various forms of cancer (Varghese, 2002).
Pathology
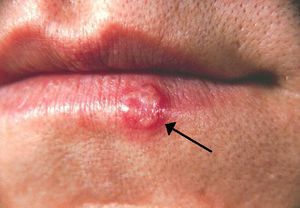
HSV-1 and HSV-2 are both transmitted through skin-to-skin contact or through contact with an infected person’s bodily fluids. HSV-1 primarily infects the areas of the mouth, throat, face, eyes, and the central nervous system, while HSV-2 primarily infects the genitals. However, both variants can infect all areas (Gupta, 2007). Transmission can also be vertical through pregnancy, and neonatal HSV infection is more severe than childhood or adult. Once infected, HSV causes blisters on the skin that are filled with active virus particles. Contact with these blisters carries the highest risk of transmission. However, these blisters are not permanent, lasting typically a few weeks. The infection then goes into a latent stage, where the infected person is asymptomatic. Though asymptomatic, the infected person may still transmit the virus. During the latent stage, HSV invades peripheral neurons and remains in the nuclei of these cells (Gupta, 2007). Eventually, the virus is triggered and re-infection begins, as virions move out of neuronal nuclei and out to re-infect epithelial areas. It is unknown exactly what triggers the resurgence of the virus. Interestingly, once infected other areas become resistant to infection: If an oral infection of HSV-1 occurs, then infections will not occur on the fingers (Gupta, 2007). Also, contracting one variant confers some protection against the other, as those infected with HSV-1 are somewhat resistant to HSV-2, while those infected with HSV-2 are resistant to HSV-1 (Gupta, 2007). Regardless of which strain is contracted, the immune system never eradicates the infection. Re-activations of the virus often become less severe and more infrequent over time, with some infected individuals becoming completely asymptomatic (Gupta, 2007).
Cell Cycle
Include some current research, with at least one figure showing data.
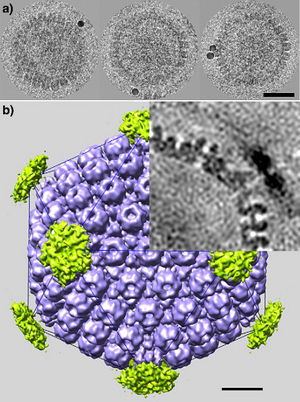
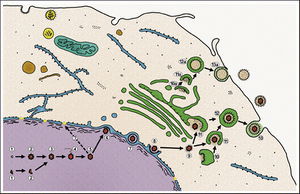
Section 3
Include some current research, with at least one figure showing data.
Conclusion
Overall text length at least 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.
