Anammox
Introduction
Anaerobic ammonium oxidation (Anammox) is a significant component of the biogeochemical nitrogen cycle [2]. The process was discovered when it was noticed that ammonium was being converted to dinitrogen in a fluidized-bed reactor system at a yeast factory nearly 20 years ago [11]. Anammox-capable bacteria are obligately anaerobic chemolithoautotrophs [3]. Five “Candidatus” anammox genera have been identified so far: “Ca. Kuenenia”, “Ca. Brocadia”, “Ca. Anammoxoglobus”, “Ca. Jettenia”, and “Ca. Scalindua” [11]. These genera make up a clade inside the phylum Planctomycetes [12]. Anammox bacteria can grow at temperatures ranging from -2 to 43 °C [2] and pH levels of 6.7 to 8.3 [3]; optimal pH is 8, while optimal temperature varies according to species [3]. These bacteria grow slowly, with a doubling time ranging from 10 days [3] to 2 weeks [11], and possess physiological adaptations unlike what would be normally expected of prokaryotes [11]. An unusual characteristic of anammox metabolism is the production of the metabolic intermediate hydrazine [11], which is one of the strongest reducing agents known in biological systems [3]. It is used as rocket fuel and in the manufacture of explosives and pesticides [6]. Anammox bacteria cannot be successfully grown in pure culture, especially due to their slow growth rate [11]; using a sequence batch reactor has proven to be a viable method of culturing them in large quantities [3].
Molecular Process of the Anammox Reaction
The overall reaction for the Anammox Process is given below [4]:
This reaction may be further broken down into the redox half-reactions that occur within the cell [4]:
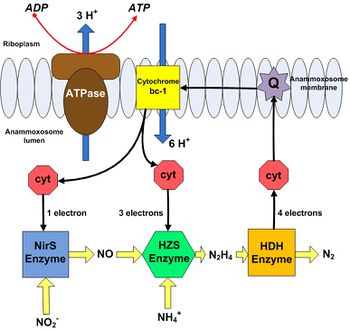
These catabolic reactions occur within the anammoxosome, a specialized pseudo-organelle within the bacterium, and create a proton gradient across the anammoxosome membrane [12]. The first step involves the reduction of nitrite to nitric oxide by nitrate reductase (NirS). Ammonium is then combined with nitric oxide by hydrazine hydrolase (HZS) to form hydrazine [12]. Soluble cytochrome-c proteins in the anammoxosome lumen provide the electrons required for these two steps [11]. The final step involves oxidation of hydrazine to dinitrogen gas via hydrazine/hydroxylamine oxidoreductase (HDH) [12]. The electrons liberated by hydrazine oxidation are transferred to another 4-electron cytochrome-c protein, which in turn delivers these electrons to ubiquinone present in the anammoxosome membrane. Ubiquinone then transfers these electrons to cytochrome–bc1 in the membrane, which then transfers them to the soluble cytochrome-c proteins that originally supplied NirS and HZS with electrons, restoring them as electron donors. Cytochrome–bc1 is a proton pump, translocating 6 protons into the anammoxosome lumen for every 4 electrons transferred [11]. This results in a buildup of protons inside the anammoxosome, which can be used to synthesize ATP [11].
Affectors of Metabolism
The anammox process is reversibly inhibited by a dissolved oxygen concentration over 2μM [3]. Additionally, despite nitrite being a substrate for anammox bacteria, a concentration greater than 10mM slows down various aspects of cellular metabolism, and a concentration greater than 20mM completely (but reversibly) stops cellular metabolism [3]. These values were obtained by observing anammox bacteria behavior in large batch cultures relative to non-inhibited control cultures [9]. Anammox bacteria that are inactive may be brought back to normal by adding catalytic amounts of hydrazine or hydroxylamine to the culture medium [9]. Hydrazine present in the growth medium has been noted to significantly reduce the long-term viability of anammox bacteria, despite being an intermediate in the anammox process [6].
Metabolic Diversity
Some diversity has been observed with respect to what the anammox bacteria can use as electron sources or acceptors. Some members of the anammox bacteria have been shown to respire iron (III) or manganese (IV) oxides, with formate as the electron donor [7]. Two species of anammox bacteria, Candidatus Anammoxoglobus propionicus and Candidatus Brocadia fulgida, are able to link the oxidation of propionate and acetate (respectively) to carbon dioxide with the reduction of nitrite to dinitrogen, instead of ammonia oxidation [3]. Certain members of the anammox bacteria are able to use methylamine or dimethylamine as electron donors in place of hydrazine [3]. The ability of anammox bacteria to use varying organic acids and amines as electron donors, as well as inorganic metallic oxides as electron acceptors in place of ammonia likely stems from incomplete respiratory systems that have been found on the genomes of the anammox bacteria [7]. These “bits and pieces” of other systems may allow molecules that would not normally be used in anammox to contribute to the existing model of the anammox process [7]. Studies based on rRNA genes reveal that anammox bacteria evolved from a common ancestor, and pathways were affected as the bacteria adapted for anammox [7].
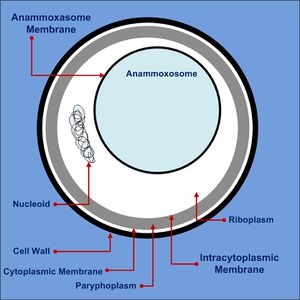
Cell Biology
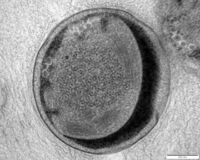
Anammox bacteria exist as coccoid cells, with a diameter of approximately 800-1100nm. They possess a proteinaceous cell wall with no peptidoglycan and have no outer membrane. As such, they do not resemble either gram-positive or gram-negative bacteria [11]. The composition of their membrane lipids is very interesting, as both ester-linked and ether-linked fatty acids are present [11]. The presence of the latter is unusual, since it is typically an Archaeal characteristic [11]. The anammox bacteria possess membrane lipids called ladderanes, which have structures not seen elsewhere in prokaryotes [1]. These special lipids make cellular membranes denser and stiffer, decreasing permeability and unwanted loss of metabolites [11]. Ladderanes reside mostly in the anammoxosome membrane, but also exist in the intracytoplasmic and cytoplasmic membranes [1].
The Anammoxosome
The anammoxosome constitutes 50-70% of the total cell volume [3], and is where all catabolic processes of anammox metabolism occur, paralleling in function to eukaryotic mitochondria [11]. It possesses many invaginations, which maximize surface area and facilitate protein localization [11]. Anammox metabolism is slow, resulting in slow translocation of protons into the anammoxosome [11]. Hydrazine made inside the anammoxosome readily traverses biological membranes and must be kept inside the anammoxosome, as it is a key metabolic intermediate [11] and has the ability to damage DNA [5]. The ladderane lipids in the anammoxosome membrane decrease it’s permeability to protons and hydrazine, ensuring they stay within the anammoxosome [11].
The Riboplasm
The riboplasm in anammox bacteria is analogous to the cytoplasmic compartment of a bacterial cell. It is where the nucleoid and ribosomes are located, and is the location of transcription and translation [11]. It is bound by the intracytoplasmic membrane and is the site of glycogen storage [12]. Glycogen provides the cell with energy and acts as a carbon source when the cell is stressed, while also playing a role in biofilm formation [12].
The Paryphoplasm
The paryphoplasm is the outermost cellular compartment in anammox bacteria, bound by the cytoplasmic membrane [12] which defines the cell boundary [3]. Ladderane lipids in the cytoplasmic membrane may provide additional strength [11]. The role of the paryphoplasm and whether it functions as periplasmic of cytoplasmic space is still the subject of research [11].
Ecological Significance
The anammox process plays a significant role in the nitrogen cycle, turning over nitrite and ammonia to dinitrogen [11]. The anammox process is one of two microbial processes that release fixed nitrogen from the environment as dinitrogen, the other being denitrification [4]. Anammox bacteria have been discovered in many environments, including suboxic marine zones, coastal sediments, lakes, and wastewater treatment plants [11]. Recently, they have been discovered in freshwater sediments [8]. Anammox is estimated to contribute up to 50% of the dinitrogen production from marine environments, resulting in the removal of fixed nitrogen [7].
Applications
One of the most significant and widespread uses of the anammox reaction is the treatment of sewage and wastewater to remove ammonium [10]. The complete oxidation of ammonia to dinitrogen is desired in wastewater treatment. While this by itself is thermodynamically possible, it is biochemically impossible [10]. Coupling the anammox reaction to aerobic oxidation of nitrate to nitrite achieves this desired effect, as the nitrite produced by aerobic ammonium oxidation is able to seed the anammox reaction, which turns over nitrite and ammonium to dinitrogen [10]. This reaction coupling is implemented using either a one-vessel system, where both reactions occur in the same container, or a two-vessel system, where the two reactions occur in separate containers [10]. Commonly treated waste-laden waters include sewage and effluent from food processing plants [10].
References
[1] Damste J.S.S., Strous, M., Rijpstra, W.I.C., Hopmans, E.C., Geenevasen, J.A.J., van Duin, A.C.T., van Niftrik, L.A., Jetten, M.S.M. “Linearly concatenated cyclobutane lipids form a dense bacterial membrane.” Nature, 2002, DOI: 10.1038/nature01128
[2] Jetten M.S.M., van Niftrik, L., Strous, M., Kartal, B., Keltjens, J.T., den Camp, H.J.M.O. “Biochemistry and molecular biology of anammox bacteria.” Critical Reviews in Biochemistry and Molecular Biology, 2009, DOI: 10.1080/10409230902722783
[3)] Kartal B., Keltjens, J.T., Jetten, M.S.M. “Metabolism and Genomics of Anammox Bacteria.” Nitrification, in press, 2011(b). ASM Press., Washington, DC.
[4] Kartal B., Maalcke, W.J., de Almeida, N.M., Cirpus, I., Gloerich, J., Geerts, W., den Camp, H.J.M.O., Harhangi, H.R., Janssen-Megens, E.M., Francoijs, K., Stunnenberg, H.G., Keltjens, J.T., Jetten, M.S.M., Strous, M. “Molecular mechanism of anaerobic ammonium oxidation.” Nature, 2011(a), DOI: 10.1038/nature.10453
[5] Parodi S., Flora, S.D., Cavanna, M., Pino, A., Robbiano, L., Bennicelli, C., Brambilla, G. “DNA-damaging Activity in Vivo and Bacterial Mutagenicity of Sixteen Hydrazine Derivatives as Related Quantitatively to their Carcinogenicity.” Cancer Research, 1981, 41: 1469 – 1482.
[6] Schalk J., Oustad, H., Kuenen, J.G., Jetten, M.S.M. “The anaerobic oxidation of hydrazine: a novel reaction in microbial nitrogen metabolism.” FEMS Microbiology Letters, 1998, 158:61– 67.
[7] Strous M., Pelletier, E., Mangenot, S., Rattei, T., Lehner, A., Taylor, M.W., Horn, M., Daims, H., Bartol-Mavel, D., Wincker, P., Barbe, V., Fonknechten, N., Vallenet, D., Segurens, B., Schenowitz-Truong, C., Medigue, C., Collingro, A., Snel, B., Dutilh, B.E., den Camp, H.J.M.O., var der Drift, C., Cirpus, I., van de Pas-Schoonen, K.T., Harhangi, H.R., van Niftrik, L., Schmid, M., Keltjens, J., van de Vossenberg, J., Kartal, B., Meier, H., Frishman, D., Huynen, M.A., Mewes, H., Weissenbach, J., Jetten, M.S.M., Wagner, M., Le Paslier, D. 2006. “Deciphering the evolution and metabolism of an anammox bacterium from a community genome.” Nature, 2006, DOI: 10.1038/nature04647
[8] Strous M., Jetten, M.S.M. “Anaerobic Oxidation of Methane and Ammonium.” Annu. Rev. Microbiol., 2004, DOI: 10.1146/annurev.micro.58.030603.123605
[9] Strous M., Kuenen, J.G., Jetten, M.S.M. “Key Physiology of Anaerobic Ammonium Oxidation.” Applied Environmental Microbiology, 1999, 65(7): 3248.
[10] van der Star W.R.L., Abma, W.R., Kartal, B., van Loosdrecht, M.C.M. “Application Of The Anammox Process.” Nitrification, in press, 2011. ASM Press., Washington, DC.
[11] van Niftrik L., Jetten, M.S.M. “Anaerobic Ammonium Oxidizing Bacteria: Unique Microorganisms with Exceptional Properties.” Microbiology and Molecular Biology Reviews, 2012, DOI: 10.1128/MMBR.05025-11
[12] van Niftrik L., Geerts, W.J.C., van Donselaar, E.G., Humbel, B.M., Webb, R.I., Fuerst, J.A., Verkleij, A.J., Jetten, M.S.M., Strous, M. “Linking Ultrastructure and Function in Four Genera of Anaerobic Ammonium-Oxidizing Bacteria: Cell Plan, Glycogen Storage, and Localization of Cytochrome c Proteins.” Journal of Bacteriology, 2008, DOI: 10.1128/JB.01449-07