Actinobacillus actinomycetemcomitans: Difference between revisions
No edit summary |
|||
| (39 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
| Line 5: | Line 7: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria; Proteobacteria; Gammaproteobacteria; Pasteurellales; Pasteurellaceae | Bacteria; Proteobacteria; Gammaproteobacteria; Pasteurellales; Pasteurellaceae | ||
===Species=== | ===Species=== | ||
| Line 14: | Line 16: | ||
|} | |} | ||
''Actinobacillus actinomycetemcomitans'' | |||
==Description and significance== | ==Description and significance== | ||
Actinobacillus actinomycetemcomitans is one of the most completely studied periodontal bacteria. It stays in the periodontal pocket of the oral cavity and damages tooth supporting tissues. See Pathology section for more details ( | ''Actinobacillus actinomycetemcomitans'' is one of the most completely studied periodontal bacteria. It stays in the periodontal pocket of the oral cavity and damages tooth supporting tissues. See Pathology section for more details (14). | ||
Actinobacillus actinomycetemcomitans is a gram negative bacterium which is spherical or rod-shaped ( | ''Actinobacillus actinomycetemcomitans'' is a gram negative bacterium which is spherical or rod-shaped (9). It’s a facultative anaerobe which can grow under either aerobic or anaerobic conditions (12). ''Actinobacillus actinomycetemcomitans'' is a typical cause of periodontitis but it may also be related to systemic infections and arterial plaques. Isolated ''Actinobacillus actinomycetemcomitans'' from periodontitis patients releases leukotoxin which kills T cells by some pathways (10). This will be discussed later in Pathology section. | ||
A complete genomic sequence of Actinobacillus actinomycetemcomitans is available in publication by David Dyer, Bruce Roe and colleagues at the University of Oklahoma. | A complete genomic sequence of ''Actinobacillus actinomycetemcomitans'' is available in publication by David Dyer, Bruce Roe and colleagues at the University of Oklahoma. | ||
[[image:hmk001.jpg]] | |||
[Image of Actinobacillus actinomycetemcomitans colony grown on selective agar from UCL Eastman Dental Institute.] | |||
==Genome structure== | ==Genome structure== | ||
The entire DNA genome of the Actinobacillus actinomycetemcomitans bacteriophage AaΦ23 was sequenced by using the shotgun sequencing ( | The entire DNA genome of the ''Actinobacillus actinomycetemcomitans'' bacteriophage AaΦ23 was sequenced by using the shotgun sequencing (17). Linear DNA contained in the phage particles is circularly mixed and abundant in the end. Therefore, the entire DNA genome structure is circular. Its size is 43,033 bp with an overall molar G+C content of 42.5 mol%. Sixty-six potential open reading frames (ORFs) were found. This includes an ORF resulting from a translational frameshift, meaning if a ribosome changes frame when translating the genetic code (22). 23 of ORFs have a putative function. Twenty-three other ORFs are homologous with other bacteria. 20 ORFs came out to be specific to the phage AaΦ23. The organization of the phage genome and several genetic functions share extensive similarities with lambdoid phages (a large group of phages). However, AaΦ23 encodes a DNA adenine methylase, and the DNA packaging strategy is more closely related to the P22 system. The attachment sites of AaΦ23 (attP) and several ''A. actinomycetemcomitans'' hosts (attB) are 49 bp long (17). | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
''Actinobacillus actinomycetemcomitans'' was separated from periodontitis patients and grown in a specific condition to characterize energy metabolism of ''A. actinomycetemcomitans''. It was grown in fructose-limited chemostat cultures under anaerobic [redox potential (Eh)<-400 mV] and microaerobic (Eh=-200 mV) conditions. In a controlled medium containing 5.2 mM K+ and 24 mM Na+, the growth rate of fructose is higher under the microaerobic condition. However, when we count the ATP yield from fermentation, the overall value of microaerobic condition is lower than the anaerobic condition. We also need to count ATP production from the respiration (13). Therefore, the total ATP production of both conditions is relatively similar which enables ''A. actinomycetemcomitans'' to grow under either aerobic or anaerobic conditions. Additionally, there is a comparison of cell growth among different concentration of media. As a result, the higher concentrations of extracellular K+ are required for rapid growth of ''A. actinomycetemcomitans'' (13). | |||
''Actinobacillus actinomycetemcomitans'' is a gram negative bacterium. There are some significant aspects of gram negative characteristic. ''A. actinomycetemomitans'' contains the polysaccharide region of lipopolysaccharide (LPS). From the LPS region, the structure of the O antigen is identified by analyzing the aqueous phase LPS from a phenol-water extract of ''A. actinomycetemcomitans'' (15). | |||
Interestingly, even though ''A. actinomycetecomitans'' is gram negative, peptidoglycans can be separated by boiling in 4% sodium dodecyl sulfate and by digestion with pronase, trypsin and alpha-amylase. This indicates that peptidoglycans may eventually be responsible for destruction of periodontal tissues (19). | |||
''Actinobacillus actinomycetemcomitans'' grows well at 37°C in 5% CO2 in air. Cells that are freshily isolated from patients have fimbriae and inner star-shaped figure (18). The fimbriae are used for the adherence and colonization of the microorganism to the oral cavity. However, when ''A. actinomycetemcomitans'' is cultured in the laboratory, it lacks star-shaped figure and fimbriae (4). | |||
==Ecology== | ==Ecology== | ||
Biofilms are populations of microorganisms that are concentrated at interfaces. Many bacterial floras grow as bioflims on various surfaces including human body parts. Oral cavity would be the one great example. In the oral cavity, biofilms are differ in thickness. Biofilms on Hard structure like tooth surfaces forms several cell layers thick, whereas biofilms of the mucosal surface is one layer thick (7). If predominating organisms are isolated from biofilms of healthy individual, the organism mainly would be Gram-positive bacteria like ''streptococci'' , ''Veillonella spp.'' , and ''Peptostreptococcus spp.''. Gram-negative bacteria can also be found but they are fewer in number (11). When periodontitis is developed in an individual, the proportions of Gram-positive and Gram-negative bacteria significantly change. Gram-negative bacteria such as ''Actinobacillus actinomycetemcomitans'' , ''Porphyromonas gingivalis'' ,and ''Tannerella forsythensis'' dominate the biofilm. Even though periodontitis probably requires association of several microorganisms, ''A. actinomycetemcomitans'' , ''P. gingivalis'' , and '' T. forsythensis'' solely develop periodontitis (2,8). | |||
==Pathology== | ==Pathology== | ||
Periodontitis | |||
''Actinobacillus actinomycetemcomitans'' is the major cause of periodontitis. Kaplan and his collegues found that ''Actinobacillus actinomycetemcomitans'' strains | |||
comprised three major phylogenetic lineages suggesting it carries virulence potential (6). Periodontitis is a bacterial infection of tooth-supporting tissues which may lead to loss of teeth. It is most common bacterial infection among middle aged people and elderly people. Also, periodontitis is considered an indicator of systemic diseases (1). | |||
Good oral hygiene is required to prevent periodontitis. To treat periodontitis, a dental hygienist or periodontist should use professional scraping tools, such as scalers and currettes to scrape off bacterial plaque around teeth and below the gum-line. Since ''A. actinomycetemcomitans'' attacks tissues and bones altogether, bone grafting surgery may be tried but there are some cases of horizontal defects which make surgery helpless. Sometimes, dentists introduce antibiotics underneath the gumline in affected areas (21). | |||
Cardiovascular diseases | |||
Chronic dental infections, such as periodontitis, increase the risk for cardiovascular disease. The mechanism between periodontitis and cardiovascular diseases are only partly understood, but here are some clues. Lipopolysaccharide of ''Actinobacillus actinomycetemcomitans'' modifies low-density lipoprotein which eventually helps accumulation of cholesterol with a support of macrophagederived foam cells (8,23). The macrophagederived foam cells are from local inflammatory response of periodontitis. Also, high-density lipoprotein, preventing oxidation of low-density lipoprotein, reversing cholesterol transport and neutralizing LPS in the circulation, is low in concentration among periodontitis patients. Therefore, low level of high-density lipoprotein causes accumulation of cholesterol (8,16). | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
There is no known benefit for this organism. | |||
==Current Research== | ==Current Research== | ||
Molecular cloning of the ''fur'' gene from ''Actinobacillus actinomycetemcomitans'' is being done by Dr. Haraszthy and others from University of Pennsylvania. Several bacterial species express virulence factor through the ''fur'' gene including ''A. actinomycetemcomitans'' and ''Escherichia coli''. The ''A. actinomycetemcomitans'' ''fur'' gene was cloned by utilizing the ''fur'' mutant in ''E. coli''. The researchers figured out that the ''fur'' is widely distributed in ''A. actinomycetemcomitans''.They suggested that further characterization of the ''fur'' gene in ''A. actinomycetemcomitans'' may improve our understanding of its role in periodontal disease (3). | |||
There was a study that leukotoxin from ''Actinobacillus actinomycetemcomitans'' causes secretion of cytokine interleukin (IL-1) from macrophages. This study mainly focused on comparing the prevalence of systemic antibodies to ''A. actinomycetemcomitans'' leukotoxin in stroke cases. The result is that more antibodies are found among women when compared to men, and women have decreased risk for stroke. The researchers stated that further studies are needeed to explain the mechanisms of their finding (5). | |||
There is a hypothesis that T-cell regulation might affect ''Actinobacillus actinomycetemcomitans'' and prevent periodontal disease. Yamashita and others isolated ''A. actinomycetemcomitans''-specific T-cell clones from the organ of rats. These clones are transferred to first group of rats and second group received no clones. Then, these two groups are orally infected with ''A. actinomycetemcomitans''. Significantly higher numbers of lymphocytes were recovered from the gingival tissue of the first group. Also, the bone loss of the first group is lower than the second group (24). These results support the hypothesis, but more research needs to be done. | |||
==References== | ==References== | ||
Edited by student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | [1] American Academy of Periodontology. Consensus report. Ann Periodontol. 1999; 4: 53. | ||
[2] Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol | |||
2000. 1994;5:78-111. | |||
[3] Haraszthy, V. I., Lally E. T., Haraszthy, G. G. & Zambon, J. J (2002). Molecular cloning of the ''fur'' gene from ''Actinobacillus actinomycetemcomitans''. ''Infect Immun'' 70, 3170-3179. | |||
[4] Inouye T, Ohta H, Kokeguchi S, Fukui K, Kato K. Colonial variation and fimbriation of Actinobacillus | |||
actinomycetemcomitans. FEMS Microbiol Lett. 1990;57:13-17. | |||
[5] Johansson, A, Johansson I., Eriksson, M., Ahren, A.M. (2005). Systemic Antibodies to the Leukotoxin of the Oral Pathogen ''Actinobacillus actinomycetemcomitans'' Correlate Negatively with Stroke in Women. Cerebrovascular Diseases 2005; 20; 226-232. | |||
[6] Kaplan, J. B., H. C. Schreiner, D. Furgan, and D.H, Fine. 2002. Population structure and genetic diversity of ''Actinobacillus actinomycetemcomitans'' strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40: 1181-1187. | |||
[7] Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev | |||
Microbiol. 2000;54:413-437. | |||
[8] Lakio, Laura. Evolutionary lineages of ''Actinobacillus actinomycetemcomitans'' bear diverse traits to support roles as a member of normal flora and as a pathogen. Accessed August 28, 2007, <http://ethesis.helsinki.fi/julkaisut/laa/hamma/vk/lakio/evolutio.pdf>. | |||
[9] Los Alamos National Laboratory: ''Actinobacillus actinomycetemcomitans'' database overview, Accessed August 25, 2007, <http://www.oralgen.lanl.gov/oralgen/bacteria/aact/>. | |||
[10] Mangan, D. F., Taichman, Lally, Wahl, (1991). Lethal effects of ''Actinobacillus actinomycetemcomitans'' leukotoxin on human T lymphocytes. ''Infect Immun'' 59: 3267-3272. | |||
[11] Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol | |||
Mol Biol Rev. 1998;62:71-109. | |||
[12] MedicineNet: Definition of Faculative, Accessed August 25, 2007, <http://www.medterms.com/script/main/art.asp?articlekey=31986>. | |||
[13] Ohta, Hiroyuki, Inoue, Fukui, (2001). Energy metabolism of ''Actinobacillus actinomycetemcomitans'' during anaerobic and microaerobic growth in low- and high- potassium continuous culture. Microbiology 2001. 147, 2461-2468. | |||
[14] Paju, Susanna, (2000). Virulence-Associated Characteristics Of ''Actinobacillus actinomycetemcomitans'', AN ORAL AND PATHOGEN, Accessed August 25, 2007, <http://ethesis.helsinki.fi/julkaisut/laa/hamma/vk/paju/virulenc.pdf>. | |||
[15] Perry, MB, LL Maclean, R Gmur, ME Wilson, (1996). Characterization of the O-polysaccharide Structure of lipopolysaccharide from ''Actinobacillus actinomycetemcomitans'' serotype b. ''Infect Immun''. 1996 April; 64(4): 1215-1219. | |||
[16] Pussinen,PJ, Jauhiainen M, Vikuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004C; 45: 139-147. | |||
[17] Resch, Gregory, Kulik, Dietrich, Meyer, (2004). Complete Genomic Nucleotide Sequence of the Temperate Bacteriophage Aa23 of ''Actinobacillus actinomycetemcomitans''. Journal of Bacteriology. 2004 August; 186(16): 5523-5528. | |||
[18] Scannapieco FA, Millar SJ, Reynolds HS, Zambon JJ, Levine MJ. Effect of anaerobiosis on the surface | |||
ultrastructure and surface proteins of Actinobacillus actinomycetemcomitans (Haemophilus | |||
actinomycetemcomitans). Infect Immun. 1987;55:2320-2323. | |||
[19] Shigaku, Kanagawa, (1989). Chemical structure and immunomodulating activities of peptidoglycan from ''Actinobacillus actinomycetemcomitans''. Accessed August 27, 2007, <http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=2489655&dopt=AbstractPlus>. | |||
[20] Slots J, Reynolds HS, Genco RJ. Actinobacillus actinomycetemcomitans in human periodontal disease: A | |||
cross-sectional microbiological investigation. Infect Immun. 1980;29:1013-1020. | |||
[21] Wikipedia: Periodontitis, Accessed on August 28, 2007, <http://en.wikipedia.org/wiki/periodontal_disease>. | |||
[22] Wikipedia: Translational frameshift, Accessed August 26, 2007, <http://en.wikipedia.org/wiki/Translational_frameshift>. | |||
[23] Yamada, Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998; 54: 628-640. | |||
[24] Yamashita, K., JW, Eastcott, MA, Taubman, DJ, Smith, Cox DS, (1991). Effect of adoptive transfer of cloned ''Actinobacillus actinomycetemcomitans'' -specific T helper cells on periodontal disease. ''Infect Immun''. 1991 Apr; 59(4): 1529-34. | |||
Edited by Hae Min Kim student of [mailto:ralarsen@ucsd.edu Rachel Larsen] | |||
Edited by KLB | |||
Latest revision as of 20:41, 10 September 2010
A Microbial Biorealm page on the genus Actinobacillus actinomycetemcomitans
Classification
Higher order taxa
Bacteria; Proteobacteria; Gammaproteobacteria; Pasteurellales; Pasteurellaceae
Species
|
NCBI: Taxonomy |
Actinobacillus actinomycetemcomitans
Description and significance
Actinobacillus actinomycetemcomitans is one of the most completely studied periodontal bacteria. It stays in the periodontal pocket of the oral cavity and damages tooth supporting tissues. See Pathology section for more details (14).
Actinobacillus actinomycetemcomitans is a gram negative bacterium which is spherical or rod-shaped (9). It’s a facultative anaerobe which can grow under either aerobic or anaerobic conditions (12). Actinobacillus actinomycetemcomitans is a typical cause of periodontitis but it may also be related to systemic infections and arterial plaques. Isolated Actinobacillus actinomycetemcomitans from periodontitis patients releases leukotoxin which kills T cells by some pathways (10). This will be discussed later in Pathology section.
A complete genomic sequence of Actinobacillus actinomycetemcomitans is available in publication by David Dyer, Bruce Roe and colleagues at the University of Oklahoma.
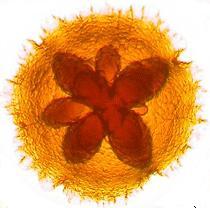 [Image of Actinobacillus actinomycetemcomitans colony grown on selective agar from UCL Eastman Dental Institute.]
[Image of Actinobacillus actinomycetemcomitans colony grown on selective agar from UCL Eastman Dental Institute.]
Genome structure
The entire DNA genome of the Actinobacillus actinomycetemcomitans bacteriophage AaΦ23 was sequenced by using the shotgun sequencing (17). Linear DNA contained in the phage particles is circularly mixed and abundant in the end. Therefore, the entire DNA genome structure is circular. Its size is 43,033 bp with an overall molar G+C content of 42.5 mol%. Sixty-six potential open reading frames (ORFs) were found. This includes an ORF resulting from a translational frameshift, meaning if a ribosome changes frame when translating the genetic code (22). 23 of ORFs have a putative function. Twenty-three other ORFs are homologous with other bacteria. 20 ORFs came out to be specific to the phage AaΦ23. The organization of the phage genome and several genetic functions share extensive similarities with lambdoid phages (a large group of phages). However, AaΦ23 encodes a DNA adenine methylase, and the DNA packaging strategy is more closely related to the P22 system. The attachment sites of AaΦ23 (attP) and several A. actinomycetemcomitans hosts (attB) are 49 bp long (17).
Cell structure and metabolism
Actinobacillus actinomycetemcomitans was separated from periodontitis patients and grown in a specific condition to characterize energy metabolism of A. actinomycetemcomitans. It was grown in fructose-limited chemostat cultures under anaerobic [redox potential (Eh)<-400 mV] and microaerobic (Eh=-200 mV) conditions. In a controlled medium containing 5.2 mM K+ and 24 mM Na+, the growth rate of fructose is higher under the microaerobic condition. However, when we count the ATP yield from fermentation, the overall value of microaerobic condition is lower than the anaerobic condition. We also need to count ATP production from the respiration (13). Therefore, the total ATP production of both conditions is relatively similar which enables A. actinomycetemcomitans to grow under either aerobic or anaerobic conditions. Additionally, there is a comparison of cell growth among different concentration of media. As a result, the higher concentrations of extracellular K+ are required for rapid growth of A. actinomycetemcomitans (13).
Actinobacillus actinomycetemcomitans is a gram negative bacterium. There are some significant aspects of gram negative characteristic. A. actinomycetemomitans contains the polysaccharide region of lipopolysaccharide (LPS). From the LPS region, the structure of the O antigen is identified by analyzing the aqueous phase LPS from a phenol-water extract of A. actinomycetemcomitans (15). Interestingly, even though A. actinomycetecomitans is gram negative, peptidoglycans can be separated by boiling in 4% sodium dodecyl sulfate and by digestion with pronase, trypsin and alpha-amylase. This indicates that peptidoglycans may eventually be responsible for destruction of periodontal tissues (19).
Actinobacillus actinomycetemcomitans grows well at 37°C in 5% CO2 in air. Cells that are freshily isolated from patients have fimbriae and inner star-shaped figure (18). The fimbriae are used for the adherence and colonization of the microorganism to the oral cavity. However, when A. actinomycetemcomitans is cultured in the laboratory, it lacks star-shaped figure and fimbriae (4).
Ecology
Biofilms are populations of microorganisms that are concentrated at interfaces. Many bacterial floras grow as bioflims on various surfaces including human body parts. Oral cavity would be the one great example. In the oral cavity, biofilms are differ in thickness. Biofilms on Hard structure like tooth surfaces forms several cell layers thick, whereas biofilms of the mucosal surface is one layer thick (7). If predominating organisms are isolated from biofilms of healthy individual, the organism mainly would be Gram-positive bacteria like streptococci , Veillonella spp. , and Peptostreptococcus spp.. Gram-negative bacteria can also be found but they are fewer in number (11). When periodontitis is developed in an individual, the proportions of Gram-positive and Gram-negative bacteria significantly change. Gram-negative bacteria such as Actinobacillus actinomycetemcomitans , Porphyromonas gingivalis ,and Tannerella forsythensis dominate the biofilm. Even though periodontitis probably requires association of several microorganisms, A. actinomycetemcomitans , P. gingivalis , and T. forsythensis solely develop periodontitis (2,8).
Pathology
Periodontitis
Actinobacillus actinomycetemcomitans is the major cause of periodontitis. Kaplan and his collegues found that Actinobacillus actinomycetemcomitans strains comprised three major phylogenetic lineages suggesting it carries virulence potential (6). Periodontitis is a bacterial infection of tooth-supporting tissues which may lead to loss of teeth. It is most common bacterial infection among middle aged people and elderly people. Also, periodontitis is considered an indicator of systemic diseases (1). Good oral hygiene is required to prevent periodontitis. To treat periodontitis, a dental hygienist or periodontist should use professional scraping tools, such as scalers and currettes to scrape off bacterial plaque around teeth and below the gum-line. Since A. actinomycetemcomitans attacks tissues and bones altogether, bone grafting surgery may be tried but there are some cases of horizontal defects which make surgery helpless. Sometimes, dentists introduce antibiotics underneath the gumline in affected areas (21).
Cardiovascular diseases
Chronic dental infections, such as periodontitis, increase the risk for cardiovascular disease. The mechanism between periodontitis and cardiovascular diseases are only partly understood, but here are some clues. Lipopolysaccharide of Actinobacillus actinomycetemcomitans modifies low-density lipoprotein which eventually helps accumulation of cholesterol with a support of macrophagederived foam cells (8,23). The macrophagederived foam cells are from local inflammatory response of periodontitis. Also, high-density lipoprotein, preventing oxidation of low-density lipoprotein, reversing cholesterol transport and neutralizing LPS in the circulation, is low in concentration among periodontitis patients. Therefore, low level of high-density lipoprotein causes accumulation of cholesterol (8,16).
Application to Biotechnology
There is no known benefit for this organism.
Current Research
Molecular cloning of the fur gene from Actinobacillus actinomycetemcomitans is being done by Dr. Haraszthy and others from University of Pennsylvania. Several bacterial species express virulence factor through the fur gene including A. actinomycetemcomitans and Escherichia coli. The A. actinomycetemcomitans fur gene was cloned by utilizing the fur mutant in E. coli. The researchers figured out that the fur is widely distributed in A. actinomycetemcomitans.They suggested that further characterization of the fur gene in A. actinomycetemcomitans may improve our understanding of its role in periodontal disease (3).
There was a study that leukotoxin from Actinobacillus actinomycetemcomitans causes secretion of cytokine interleukin (IL-1) from macrophages. This study mainly focused on comparing the prevalence of systemic antibodies to A. actinomycetemcomitans leukotoxin in stroke cases. The result is that more antibodies are found among women when compared to men, and women have decreased risk for stroke. The researchers stated that further studies are needeed to explain the mechanisms of their finding (5).
There is a hypothesis that T-cell regulation might affect Actinobacillus actinomycetemcomitans and prevent periodontal disease. Yamashita and others isolated A. actinomycetemcomitans-specific T-cell clones from the organ of rats. These clones are transferred to first group of rats and second group received no clones. Then, these two groups are orally infected with A. actinomycetemcomitans. Significantly higher numbers of lymphocytes were recovered from the gingival tissue of the first group. Also, the bone loss of the first group is lower than the second group (24). These results support the hypothesis, but more research needs to be done.
References
[1] American Academy of Periodontology. Consensus report. Ann Periodontol. 1999; 4: 53.
[2] Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111.
[3] Haraszthy, V. I., Lally E. T., Haraszthy, G. G. & Zambon, J. J (2002). Molecular cloning of the fur gene from Actinobacillus actinomycetemcomitans. Infect Immun 70, 3170-3179.
[4] Inouye T, Ohta H, Kokeguchi S, Fukui K, Kato K. Colonial variation and fimbriation of Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1990;57:13-17.
[5] Johansson, A, Johansson I., Eriksson, M., Ahren, A.M. (2005). Systemic Antibodies to the Leukotoxin of the Oral Pathogen Actinobacillus actinomycetemcomitans Correlate Negatively with Stroke in Women. Cerebrovascular Diseases 2005; 20; 226-232.
[6] Kaplan, J. B., H. C. Schreiner, D. Furgan, and D.H, Fine. 2002. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40: 1181-1187.
[7] Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413-437.
[8] Lakio, Laura. Evolutionary lineages of Actinobacillus actinomycetemcomitans bear diverse traits to support roles as a member of normal flora and as a pathogen. Accessed August 28, 2007, <http://ethesis.helsinki.fi/julkaisut/laa/hamma/vk/lakio/evolutio.pdf>.
[9] Los Alamos National Laboratory: Actinobacillus actinomycetemcomitans database overview, Accessed August 25, 2007, <http://www.oralgen.lanl.gov/oralgen/bacteria/aact/>.
[10] Mangan, D. F., Taichman, Lally, Wahl, (1991). Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun 59: 3267-3272.
[11] Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71-109.
[12] MedicineNet: Definition of Faculative, Accessed August 25, 2007, <http://www.medterms.com/script/main/art.asp?articlekey=31986>.
[13] Ohta, Hiroyuki, Inoue, Fukui, (2001). Energy metabolism of Actinobacillus actinomycetemcomitans during anaerobic and microaerobic growth in low- and high- potassium continuous culture. Microbiology 2001. 147, 2461-2468.
[14] Paju, Susanna, (2000). Virulence-Associated Characteristics Of Actinobacillus actinomycetemcomitans, AN ORAL AND PATHOGEN, Accessed August 25, 2007, <http://ethesis.helsinki.fi/julkaisut/laa/hamma/vk/paju/virulenc.pdf>.
[15] Perry, MB, LL Maclean, R Gmur, ME Wilson, (1996). Characterization of the O-polysaccharide Structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect Immun. 1996 April; 64(4): 1215-1219.
[16] Pussinen,PJ, Jauhiainen M, Vikuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004C; 45: 139-147.
[17] Resch, Gregory, Kulik, Dietrich, Meyer, (2004). Complete Genomic Nucleotide Sequence of the Temperate Bacteriophage Aa23 of Actinobacillus actinomycetemcomitans. Journal of Bacteriology. 2004 August; 186(16): 5523-5528.
[18] Scannapieco FA, Millar SJ, Reynolds HS, Zambon JJ, Levine MJ. Effect of anaerobiosis on the surface ultrastructure and surface proteins of Actinobacillus actinomycetemcomitans (Haemophilus actinomycetemcomitans). Infect Immun. 1987;55:2320-2323.
[19] Shigaku, Kanagawa, (1989). Chemical structure and immunomodulating activities of peptidoglycan from Actinobacillus actinomycetemcomitans. Accessed August 27, 2007, <http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=2489655&dopt=AbstractPlus>.
[20] Slots J, Reynolds HS, Genco RJ. Actinobacillus actinomycetemcomitans in human periodontal disease: A cross-sectional microbiological investigation. Infect Immun. 1980;29:1013-1020.
[21] Wikipedia: Periodontitis, Accessed on August 28, 2007, <http://en.wikipedia.org/wiki/periodontal_disease>.
[22] Wikipedia: Translational frameshift, Accessed August 26, 2007, <http://en.wikipedia.org/wiki/Translational_frameshift>.
[23] Yamada, Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998; 54: 628-640.
[24] Yamashita, K., JW, Eastcott, MA, Taubman, DJ, Smith, Cox DS, (1991). Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans -specific T helper cells on periodontal disease. Infect Immun. 1991 Apr; 59(4): 1529-34.
Edited by Hae Min Kim student of Rachel Larsen
Edited by KLB
