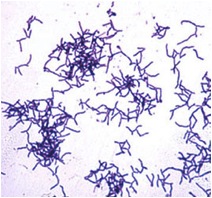
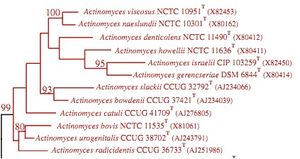
Actinomyces israelii NEUF2011
A Microbial Biorealm page on the genus Actinomyces israelii NEUF2011
Classification
Higher order taxa
Superkingdom: Bacteria Phylum: Actinobacteria Class: Actinobacteria Order: Actinomycetales Family: Actinomycetaceae Genus: Actinomyces
Use NCBI link to find]
Species
|
NCBI: Taxonomy |
Species: A. israelii
Description and significance
Actinomyces israelii is a filamentous anaerobic bacteria that is gram positive, non-spore forming, and non-acid-fast (Actinomyces israelii). It is an organism typically found in the soil and in decaying organic matter including wet hay and straw, but is can also be found in oral cavities, dental plaques and the intestinal tract of mammals (Roque, 2010). Although A. israelii is not normally a disease causing organism, it is the primary causative agent of Actinomycosis, a long term infection that commonly affects the face and neck (Dugdale, 2009). A. israelii is sensitive to penicillin, ampicillin, tetracyclines, chloramphenicol, clindamycin, and selected aminoglycosides (Actinomyces israelii).
Genome structure
Actinobacteria are categorized by their high GC content in DNA (Book). According to NCBI, the genome of A. israelii is currently being sequenced, using Actinomyces israelii F0345 for comparative genome analysis. The genome sequences is not available on NCBI, or found in published articles.
The 16S ribosomal RNA sequence in Actinomyces bacteria is used for phylogenetic analysis of the species within the genus. Analysis of the 16S ribosomal RNA sequence allows differentiation of species. This is because this sequence tends to vary between species in the genus. Literature about A. israelii currently discusses this sequence as significant, with little discussion about other gene sequences (Clinical Microbiology Review, 2004).
Cell structure and metabolism
The cell surface of A. israelii is covered with a thick and fuzzy coat. The Gram-positive cell wall is surrounded by an outer coat, with hair-like fimbriae covering the surface and extending from it (Figdor & Davies, 1997).
A. israelii is anaerobic to micro-aerobic (Porteous, 1964) and requires nicotinic acid, riboflavin, pyridoxal, and biotin as growth factors in media. The bacteria synthesize coenzyme-A de novo for metabolic function. The amino acid L-cysteine is essential to the nutrition of A. israelii (Christie & Porteous, 1962). A. israelii does not produce catalase, and does not contain CAMP factor, however the bacteria is able to reduce nitrate and hydrolyze urea, as well as ferment arabinose, maltose, sucrose, xylose, and trehalose. A. israelii produces Beta-galactosidase, meaning it is able to break down lactose (JCM, 2001).
Ecology
A. israelii is a very successful species of bacteria which can be found in a wide range of habitats including both natural and man made habitats. This bacteria is a natural component of the human gastrointestinal and oral flora (Ferrari, Couto, Murta-Oliveira, Conceição & Silva, 2000).
Also commonly found in soil, among other members of its order Actinomycetales, A. israelii have a significant role in the recycling of nutrients. This bacteria is considered a mesophile, and generally only become metabolically active in the presence of appropriate nutrients and conditions (Goodfellow & Williams, 1983). Actinomycetes, including A. israelii are thought to be very efficient in degrading polymers occurring naturally in plant litter and soil. A. israelii primarily finds means of dispersal through water movement, and arthropods (Goodfellow & Williams, 1983).
Pathology
A. israelii is the cause of 56% of actinomycotic abscess and empyema. Actinomycosis is a rare infection in humans which manifests itself in abscess formation, tissue fibrosis, sulfur granules in lesions, and the formation of drainage sinuses. The most common forms of actinomycosis are cervicofacial actinomycosis, thoracic actinomycosis, adbominopelvic actinomycosis, and central nervous system actinomycosis (Gaini, Roge, Pedersen, & Brenoe, 2006). A. israelii is able to grow in host tissue by surviving the host's defenses such as phagocytes. The bacteria do this by growing in colonies made up of cohesive branching filaments of cells, all-together forming a biofilm. Structures called fimbriae on the cell surface that are similar to, but smaller than flagella, and made of protein located on the bacterial cell surface allow A. israelii to grow in these colonies. A. israelii cells make connections to surfaces, such as tissues and teeth, as well as to one another, forming the filamentous colonies that allow the cells to avoid phagocytosis by the host immune response (Figdor & Davies, 1997).
Actinomycosis is common in women with intrauterine devices. 85% of cases of pelvic actinomycosis, a rare infection, are in women with IUD's. Though A. israelii is an organism commonly found in the vagina, an accumulation of the bacteria around a foreign object such as an IUD creates a biofilm infection that is difficult to identify and treat. Identification of A. israelii as the causative agent of these infections is often after surgery or removal of the IUD to treat the infection when sulfur granules can be seen (Iwasaki, Nishikawa, Akutagawa, Fujimoto, Teramoto, & Kudo, 2003).
Treatment of Actinomycosis caused by A. israelii is usually with antibiotics for a long period of time, up to a year. If the abscess persists after this treatment the abscess is drained surgically. Since A. israelii is usually a normal non-pathogenic organism living in the mouth, good oral hygiene can prevent some forms of Actinomycosis caused by A. israelii (Dugdale).
Current Research
A current study by Braz-Silva et al. (2010) discusses the usefulness of simple cytological techniques, such as stains, to identify the infectious agents growing in the mouth. This could help to diagnose infections like Actinomycosis caused by A. israelii sooner by using the rapid techniques such as Gram-staining. A gram stain of A. israeli would show a Gram-positive organism, highlighting the branching filaments covering the cell that would allow fast identification of the bacteria.
In another current article: (Santos & Candido Da Silva, 2010), published in the "International Journal of Medicine and Medical Sciences"A. israelii
Cool Factor
Describe something you fing "cool" about this microbe.
The predominant causative agent of human actinomycosis is Actinomyces israelii. A. israelii is able to attach and grow in synthetic intrauterine media and present on the copper surface likely due to the production of biofilm(Carrillo & Valdez, 2009).
References
Actinomyces israelii | humen health.. (n.d.). Retrieved from http://www.humenhealth.com/actinomyces-israelii
Braz-Silva P., Magalhães M., Hofman P., et al. (2010). Usefulness of oral cytopathology in the diagnosis of infectious diseases. Cytopathology, 21(5):285-299.
Carrillo, M., Valdez, B., Vargas, L., Alvarez, L., Schorr, M., Zlatev, R., & Stoytcheva, M. (n.d).(2009) In vitro Actinomyces israelii biofilm development on IUD copper surfaces. Contraception, 81(3), 261-264. .
Christie, A.O., & Porteous, J.W. (1962). The Growth Factor Requirements of the Wills Strain of Actimonyces israelii Growing in a Chemically Defined Medium. General Microbiology, 28.
Clinical Microbiology Reviews. (2004). NCBI, 17, 840–862. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC523561/pdf/0041-04.pdf
Dugdale, D.C., Vyas, J.M.. (2009). Actinomycosis. MedlinePlus, NIH. http://www.nlm.nih.gov/medlineplus/ency/article/000599.htm
Dugdale, D. C. (2009, December 1). Actinomycosis - pub med health. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001625/
Ferrari, T. A., Couto, C. A., Murta-Oliveira, C. C., Conceição, S. A., & Silva, R. G. (2000). Actinomycosis of the Colon: A Rare Form of Presentation. Scandinavian Journal of Gastroenterology, 35(1), 108-109.
Figdor, D. & Davies, J. (1997). Cell surface structures of Actinomyces israelii. Australian Dental Journal, 42:(2):125-128.
Gaini, S., Roge, B., Pedersen, C., Pedersen, S., & Brenoe, A. (2006). Severe Actinomyces israelii infection involving the entire spinal cord. Scandinavian Journal of Infectious Diseases, 38(3), 211-213.
Goodfellow, M., & Williams , S. T. (1983). Ecology of actinomyces. Ann. Rev. Microbial, (37), 189-216.
Iwasaki, M., Nishikawa, A., Akutagawa, N., Fujimoto, T., Teramoto, M., & Kudo, R. (2003). A case of ovarian actinomycosis. Infectious Diseases In Obstetrics & Gynecology, 11(3), 171-173.
Journal of Clinical Microbiology. (2001). 39,11, 3955-3961.
Porteous, J.W. (1964).The nutrition of the micro-aerophilic pathogen Actinomyces israelii. Nutrition of Microorganisms, 23.
Roque, J. M. (2010, May 10). Actinomycosis in opthalmology.. Retrieved from http://emedicine.medscape.com/article/1203061-overview
Santos, V. R., & Candido Da Silva, J. L. (2010). Experimental chronic inflammation induced in mice periodontium by actinomyces israelii entrapped in alginate gel. International Journal of Medicine and Medical Sciences, 2(10), 314-317. Retrieved from http://www.academicjournals.org/IJMMs/PDF/pdf2010/Oct/Santos et al.pdf
Schaal, K.P., Yassin A.F., Stackebrandt, E. (2006). The Family Actinomycetaceae: The Genera Actinomyces, Actinobaculum, Arcanobacterium, Varibaculum, and Mobiluncus. Prokaryotes, 3:430-537.