Adenovirus-based Gene Therapy: a Promising Novel Cancer Therapy
A Viral Biorealm page on the family Adenovirus-based Gene Therapy: a Promising Novel Cancer Therapy
Baltimore Classification
Group I, double stranded DNA
Higher order categories
Family Adenoviriade
Genera Atadenovirus, Aviadenovirus, Ichtadenovirus, Mastadenovirus, Siadenovirus
Type Species of Genera:
Genus Atadenovirus: Ovine adenovirus D
Genus Aviadenovirus: Fowl adenovirus A
Genus Ichtadenovirus: Sturgeon adenovirus A
Genus Mastadenovirus: Human adenovirus C, Human adenovirus 36
Genus Siadenovirus: Frog adenovirus
Diversity and Classification: 57 accepted human adenovirus serotypes (HAdV-1 to 57) in 7 species (A-G)
A: 12, 18, 31
B: 3, 7, 11, 14, 16, 21, 34, 35, 50, 55
C: 1, 2, 5, 6, 57 [2]
D: 8, 9, 10, 13, 15, 17, 19, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33, 36, 37, 38, 39, 42, 43, 44, 45, 46, 47, 48, 49, 51, 53, 54, 56[3]
E: 4
F: 40, 41
G: 52[4]
Background
Adenovirus (Ad) structure is characterized by its medium size (90-100nm) and non-enveloped icosahedral, including a nucleocapsid[1]. It is the largest of the non-enveloped viruses, and contains a single double stranded (ds) DNA genome ~36 to 38 kilobases in size[2]. About 57 serotypes exist in humans and cause 5-10% of upper respiratory infections in children; it is also highly prevalent among adults. Ad infects various species of vertebrates and were first found and isolated in human adenoids in the mid 1950s[1].
Ad is an extremely rigid virus, ubiquitous in human and animal populations as it can survive long periods of time out of the host and is endemic year round[3]. Infection has been found in multiple organ systems, yet most remain asymptomatic. Most adults have measurable titers of anti-Ad antibodies, indicating a prior infection or exposure[3]. The virus is oncogenic in rodents only[3].
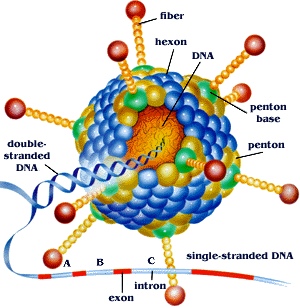
The viral capsid consists of 252 proteins, involving of three distinct types: fiber, penton based and hexon[2]. Both fiber and penton base proteins are key in receptor binding and internalization[2]. Hexon composes most of the viral capsid. The viral genome is large, consisting of a single double-stranded DNA molecule 36 to 38 kilobases in size[2]. Replication and assembly occur in the nucleus of infected cells while mature virions leave cell via disintegration[2].
Respiratory tract infections are a result from droplet inhalation, while gastrointestinal problems are due to fecal-oral transmission[4]. There are 3 different types of interactions that can occur upon infection; lytic infection occurs when the Ad enters human epithelial cells and continues through an entire replication cycle, with a host inflammatory response[4]. Chronic or latent infection can also occur, usually infecting the lymphoid tissue, and involves no external symptoms[4]; the exact mechanism is unknown. The last interaction that can occur is oncogenic within rodents, as an adenovirus integrates its own DNA into host DNA and produces E1A proteins, altering transcription, deregulating apoptosis and forming malignant tumors[4]. E1A facilitates oncogeny by activation of ras or c-src signaling, polyoma middle T protein[4]. Paradoxically, this role of E1A is not seen in humans, as Ad sequences are non-existent in human tumor cells, despite being highly prevalent infection in human population[4].
Ad genome is composed of linear, non-segmented dsDNA, size ranging between 26 and 45 Kbp[5]; the virus can contain up to 22-40 distinct genes[5]. Its genome is significantly larger than that of other viruses within its group, yet it is still considered a relatively "simple virus" and is extremely dependent on host cell for life cycle and replication process[5]. The DNA has a terminal 55 kDa protein within each of its 5' ends of its linear dsDNA which serve as primers in viral replication and ensure that all of the viral genome is replicated[5].
Adenovirus is Key Vector: Benefits and Risk Factors
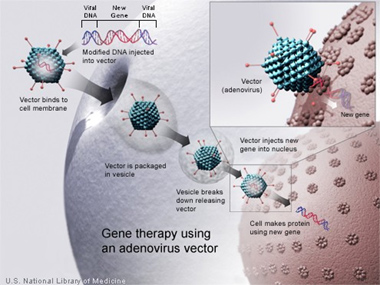
Adenovirus (Ad) based cancer gene therapy is an innovative and novel form of treatment that is used as an alternative today. Gene therapy is a new approach to traditional therapies to combat severe forms of cancer. Gene delivery is used to “correct” and rebuild broken down and infected tissue within the body[6]. Essentially, DNA is used as a "pharmaceutical agent," as DNA can supplement of alter genes within cells[6]. Most effective form of gene therapy today uses DNA that encodes form a therapeutic gene to function in the place of one that is mutated[6]. Another common form is using DNA that encodes for a protein which serves itself as a drug[6].
Ads have been isolated from a large number of different species, 100 serotypes, 57 in humans[3]. Most gene therapy involves serotypes 5 and 2[3]. Ad is highly coveted for its ability to effectively transduce cells that both dividing and non-dividing[7]. Ad Vectors also have capacity to hold large segments of DNA (7.5 kil basepairs); they are also easily manipulated with recombinant DNA techniques and has the ability to produce high titers[7]. Infection of Ad is mediated by the binding of the fiber-knob region to a receptor of target cell, the coxsackie-Ad receptor (CAR), for most serotypes in Ad, most commonly serotype 5 found in humans[7]. Entry and internalization of the virus is mediated by an interaction between the penton-base Arg-Gly-Asp (RGD) and cellular αvβ integrins, leading to the endocytosis of the viron through clathrin-coated pits[7]. The virus disassembles in the endosome and viral DNA is transported to the nucleus, resulting in expression of viral genes or transgenes[7]. Ad DNA is not integrated in the host genome so the risk of mutation is extremely low; however, it remains in an episomal state[7].
Secondary receptor interactions involving heparan sulfate proteoglycans (HSPGs), have also been reported[7]. Expression of CAR is a rate-limiting factor in infectivity with Ad, as expresion is highly variable in primary human tumor cells[7]. This loss of CAR expression may be caused by the progression of malignant tumors and their state or aggressiveness[7]. CAR is localized within tight junctions, also playing a role in cell adhesion[7].
Ad vector infection enlists a wide variety of immune response both humoral and cellular, in response to both Ad vector and already disease-infected cells[8]. Ad presentation allows for activation of the immune system and for a natural response to occur in order to combat tumor antigens[8]. However, using Ad as vectors is risky in that too high a dose will result in acute toxicity and autoimmunity[8]. To date, side effects have been mild in Ad therapy, yet non-target side effects are consistently be monitored, both long and short term in patients, in particular in the liver[8].
Researchers are currently trying to increase the Ad gene transfer vectors to target tumor cells and decrease any targeting to the liver. There are a number of methods currently being solicited including: Ad fiber pseudotyping, or an alteration of virus tropism through substitution of receptor binding-proteins with those of other serotypes[9]; for example, the substitution of Ad serotype 5 (Ad5) with a knob from Ad serotype 3 (Ad3)[9]. Experimental data with the Ad5/3 chimeras have showed enhanced infectivity in isolated cancer cells without increasing gene delivery and toxicity to murine liver[9].
Ad are the most widely solicited vectors for gene therapy, following to retroviruses[10]. Many new approaches to other targets and capsid modifactions are also being explored.
Gene Therapy
Three regions of the viral genome can accept insertions or substitutions of DNA to generate an independent therapeutic virus vector: a region in E1, E3, and a short region between E4 and end of the genome[10]. In the first-generation vectors, the E1 region was removed and replaced with a therapeutic transgene, inhibiting viral replication[10]. Viral transcription of remaining viral genes still occurred, resulting in early innate host cytokine transcription and antigen immune responses[10]. This response caused a dramatic decrease in amount of time allotted to gene expression, due to cell-mediated destruction of the transduced cells[10]. Strong immune responses have deemed many studies unsuccessful[10].
Early trials studied effect of suppression of immune response through natuarally co-administration of low-dose etoposide, a cytotoxic agent and anticancer drug, but results were met with limited success[10]. Second and third generation adenoviral vectors have deletions in E1, E2 and E4 genes, since proteins encoded within these genes induce most host immune responses[10]. These new vectors exhibit decreased toxicity and result in prolonged gene expression in in vivo studies. An important limitation in use of recombinants is difficulty in obtaining efficient gene transfer upon second administration of viral vectors because of neutralizing antibodies[10]. B-cells and T helpers solicited by the major histocompatibility complex (MHC)class II presentation of viral cannot be fixed by a re-design of vector[10]. Therefore, treatment should only be designated to cases where temporary protein expression is critical in order to stimulate the immune system against cancer or induce apoptotic stimuli, as viral DNA does not integrate within host and eventually disappears[11].
Advantages to non-host- integrated genes in the chromosome are low disturbances in genes or cellular processes within the body[10]. In preclinical trials, vectors have been found to be expressed in the liver, skeletal muscle, heart, brain, lung, pancreas and humor tissues[10]. Administration of Ad intravenously, resulted in virus accumulation in the liver[10]. Treatment close to reproductive organs was designated as safe, as no offspring exhibited germline transmission[10].
Alternative Approaches
Results so far have been unsuccessful. The first generation of Ad vectors were unable to replicate within cells and therefore maintain therapeutic effects, however, they could penetrate through cancer cells[10].
Two strategies have been used to restrict viral replication to target cells and spare normal tissue. The first method includes genetic complementation type, replication-competent oncolytic agents or conditionally replicative Ad CRAds[10]. These CRAds have a mutation in immediate early E1A or E1B Ad region, complemented in tumor cells and not in normal cells[10]; control is allocated to a tumor/tissue specific promoters.
Prototypes of type 1 CRAds are Adl1520 or ONYX-015. ONYX-015 contains two inactivating mutations in the gene encoding for E1B55k protein[10]. E1B55k protein binds and inactivates the tumor-suppressor protein p53 in humans[10]. This vector works in targeting cancer cells, only replicating in cells which express p53, the alternate reading frame p14ARF, is mutated[10].
Another type-1 CRAd is through a mutation of E1A, like Ad5Δ24 or CB016, which have specific mutations in individual domains of the protein[10]. This use of CRAd removes S phase induction of virus which is not required in proliferating tumor cells or cervical cells transformed by human papillomavirus (HPV) viruses[10].
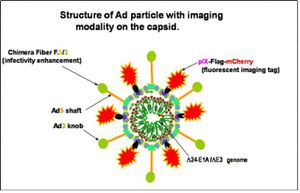
Type 2 CRAds include a tissue-specific promoter (TSP), for specific target cells[10]. Promoter fragments with specific activity used in Type 2 CRAds are rare, as structure-function relationship of promoter regions have not been well characterized[10]. TSPs have only been observed empirically, yet hold strong promise for functional therapies[10]. One of the most promising TSPs is for ovarian cancer, secretory leukoprotease inhibitor (SLPI), a 11.7kDa serine protease inhibitor, highly expressed in different human carcinomas like breast, lung, endometrium and ovarian cancer[10]. Gene expression analysis has been used to demonstrate that SLPI is 60-fold upregulated in ovarian carcinomas compared to normal ovarian epithelium, and minimally expressed in normal liver[10].
Recently a chimeric 5/3 fiber modified SLPI CRAd demonstrated a strong antitumor effect compared to wild-type Ad in murine ovarian cancer[10].
The promoter, vascular endothelial growth factor (VEGF) is effective at targeting molecule for the treatment of non-small cell lung cancer (NSCLC). VEGF is also one of the most important inducers of angiogenesis, a negative side effect in cancer involving overgrowth of new blood vessels from pre-existing vessels, which is upregulated in various cancers[10].
Midkine gene serves as a heparin-binding growth factor, and responds to retinoic acid[12]; it has been reported to be over-expressed in a large number of malignant tumors[10]. Midkine promoter has been used successfully for vector gene delivery and CRAd targeting approaches[10]. Promoters of CXC chemokine CXCR4 and Survivin, exhibit an optimal profile of activity and specificity in different tumor types[10].Genes expressing CXCR4 and Survivin play a major role in progression of metastasis in various tumors[10]. CXCR4 expression is upregulated in breast cancer cells but is not detectable in normal mammal epithelial cells. Survivin is an inhibitor of apoptosis (IAP) and expression levels of Survivin have been shown to be clinical helpful in detection of human gliomas[10]. Survivin is overexpressed in cervical intra-epithelial neoplasias (CINs) and squamous cell carcinomas (SCC) of uterine cervix[10].
Gene therapy strategies combine with a multimodality treatment have the potential to cure cancer patients. Major challenges in development include effective gene therapy vectors since vectors available currently result insufficient gene transfer. Targeting of viral vectors to cellular structures with tumor cells needs further development, in order to penetrate solid tumor masses. Current generation of replication-competent Ads demonstrate superior tumor penetration and oncolysis, compared with first generation viruses. Replication competent viruses will be paired with therapeutic transgenes, like cytokines. Deterioration of solid tumors is a process, and successful gene therapy approaches will require several rounds of viral administration, whose efficacy may be inhibited by neutralizing antibodies. Strategies to reduce effect of antibodies, include alternating related viruses with different capsids or co-treatment with immunosuppressive drugs for temporary abolition of antibodies.
