African Sleeping Sickness: Trypanosome Invasion Mechanism
Introduction
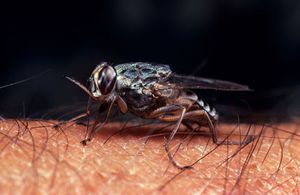
By Katie Lensmeyer
African Sleeping Sickness (Trypanosome brucei gambiense) is a microbial vector driven disease that affects many parts of Africa. The disease takes action by first invading the peripheral nervous system of its host and soon after passes the blood brain barrier to damage neurons within the brain. The result of this infection is fatal to the mammalian host. From initial infection to death of the host system, this microbe needs only a few short months to become fatal. How is it that this disease can invade such secure parts of the human system so quickly? The human immune system seems to be far to advanced to let this microbe produce the affects it does. Recent research looks deeply into both the spread of the infection through the mammalian host as well as the growth of the microbe within its vector, the tsetse fly.
The relationship between the tsetse fly and T. Brucei is that of an obligate parasite, meaning the cell needs a host cell in order to function. Once transmitted from the tsetse fly to a mammal, the microbe becomes pathogenic. Unsurprisingly, across the 36 African countries that it is found in, the tsetse fly is known to carry a variety of trypanosomiasis infections. The tsetse fly is immune to all of the trypanosome diseases that they transmit. This protection comes down to their lack of hemolymph within their blood. Their nutrient availability can no provide the microbe with the nourishment needed in order for the trypanosome cells to replicate into their pathogenic form. The cell does however have a mild affect on its fly host in the way that any normal parasite-host relationship would.
Phylogenetic reconstruction of this disease has shown that this disease has existed for approximately 300 million years. The microbial coexistence with its insect parasites has existed for equally as long. This ancient coexistence between African mammals and their blood-sucking insect counterparts is assumed to be why most native Animalia are not affected by the disease. This immunity, however, does not stretch to the domesticated animals of the area. Trypanosome brucei, the form of trypanosomes that causes sleeping sickness, is expected to be a recent development within history of trypanosomes. Most researchers suspect this because Trypanosome brucei is the only trypanosome microbe that humans are not resistant to. [1]
Sleeping Sickness is a unique microbe that, despite its phylogenetic relatives, is extremely pathogenic to humans and other mammals. What is the disease mechanism to this harmful cell? Research explored here aims to explain the inoculation of the disease within both the host and the parasite, as well as explains how the unique symptoms of the disease are produced within the infected human host.
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [2]
[3]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
What is African Sleeping Sickness?
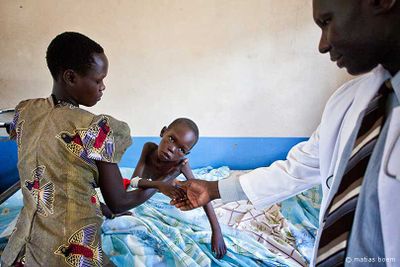
African Trypanosomiasis, or better known as African Sleeping Sickness, is a parasite driven infection of the human nervous system. The disease is caused by the microbial parasites of the species Trypanosoma brucei and than transmitted through the tsetse fly, found only in rural parts of Africa. Throughout history, this disease has been classified as a public health problem seen primarily in sub-saharan areas of Africa. About 10,000 cases of the disease are reported every year to the World Health organization, but unfortunately it is expected that most cases go unreported and/or undiagnosed.
[4]
Because this disease is vector borne, the microbe, trypanosome brucei, enters the human system by ways of the skin. An infected tsetse fly must bite the host, and through this wound the protozoan enters the system. After initial infection, the disease has two stages. The first of these stages is the time in which the parasite is found within the peripheral nervous system, but has not yet made its way into the central nervous system. The second stage begins when the infection has passed the blood brain barrier and resides within the central nervous system. The disease than acts quickly, leaving its host with symptoms of fever, tremors, swollen lymph nodes, sleep disturbances, and speech problems within the first two weeks of infection. Following weeks lead to neurological deterioration ending in coma and soon after death. An untreated case can expect the the disease to become fatal within a few months. [5]
Cell Structure and Function
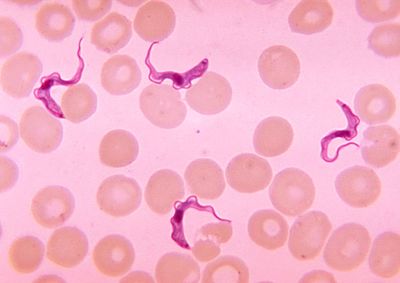
Cell Structure
Trypanosoma cells are small (approxiamtely 50um) and heterotrophic, meaning they do not generate their own food source. The shape of the cell itself is long and oval with curved edges with a strong flagellum projecting off of the back end of the cell. The cell holds its struture through the presence of a highly polarized microtubule cytoskeleton. This cytoskeleton provides defiinite locations for the cell’s organelles (i.e. the flagellar pocket, flagellum, kinetoplast, mitochondrion and nucleus) within the center and posterior ends of the cell (end opposite of the flagella). [6] This cell expresses traits within its structure that are rather unique. Analogous to what is seen in its phylum Euglenozoa, the cell has a stiffening paraxial rod within its flagellum. Similarities with members of the cells order, Kinetoplastida, exist as well. The Trypansoma cell expresses a large cluster of DNA at the opposite end of the cell from the flagellum. This clump of DNA, otherwise known as the kinetoplast, extends from the cell’s unusually long mitochondrion and functions to determine its form within its human host. [7]
Upon initial entry into the host environment, the microbe finds itself floating within the bloodstream of the mammal in which it infected. This part of the human system is flowing with host defense mechanisms, both innate and adaptive immune responses, ready to attack any intruder. Trypanosoma has evolved to travel through this environment without detection through the presence of variant surface glycoproteins (VSG) that coat its cell wall. These VSG express one of ~1500 surface glycoprotein genes. The gene used to express these proteins changes with every 100th replication cycle to ensure the infections longevity. Upon detection, the host immune system will begin launching a complimentary protection response against trypanosoma. The change in transcription of the glycoprotein genes ensures that this complementary immune response is ineffective against the pathogen because the new VSG have developed and are undetectable. This characteristic is why patients with trypanosomiasis often experience symptoms of the disease followed by a period of latency. The disease has not dissipated but rather evaded the developed defenses of the host cell. These VSG properties are only found at certain times within the cells lifecycle: when the cell is developing within the saliva of the tsetse fly and when traveling through the host's bloodstream.
Inoculation Within the Insect Vector
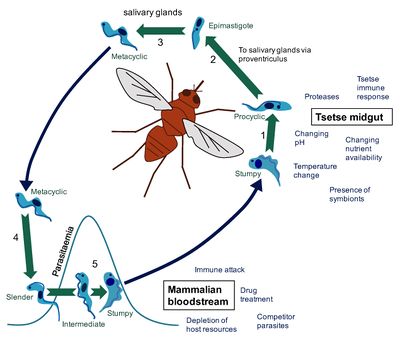
The only known vector for the disease is the tsetse fly, which can be found all over Sub-Saharan Africa. Strangely, the tsetse fly acquire the trypanosoma microbe from biting infected animals or humans who conceal pathogenic parasites. [8] Once an individual fly gains the pathogenic cell, it modifies itself into a form that can be delivered and infect human hosts. In order to transform into the correct version of itself, the cell undergoes a series of developemental cycles including cell division to prepare themselves for infection. This augmentation within the insect can be sectioned into three different stages: the procyclic form, the epimastigote form and the metacyclic form. The procyclic and epimastigote forms exist for multiplication purposes, whereas the metacyclic form functions as an adaptation period inorder to effectively integrate itself within a human host. [9]
Upon entrance into the insect from ingesting an infected human’s blood, the so called “stumpy” form of T. brucei enters and must adjust itself to survive within the environemnt of the fly. This intial transformation is composed of a varitey of reactions that are not yet fully understood. Many suspect that proteases within the insect’s midgut ignite a trigger response to the new environment. Beyond this idea, some believe that the microbe posses a cold-shock response that appears as the microbe travels from the warm-blooded human to the exothermic fly. Despite the controversy of this initial transformation, the “stumpy” form of the trypanosome remodels into the first form, the procyclic form, which than quickly attaches to the cells of the ectoperitrophic space and begin their growth into a more motile form. This form is also called the trypomastigote. The trypomastigote moves rather rapidly up the midgut of the insect and into the salivary glands. By connection to the kinetoplast, the cell enters the second, epimastigote form. This long form resides and begins replication within the salivary gland cell membranes. The cell must soon divide to form the short epimastigote form. This developmental form is currently considered to be responsible for the growth of the metacyclic trypanosomes that arre found prior to the cellular infection of a mammalian host. This cell division process is currently a popular research topic. The specifics of this differntiation is not fully understood. However, research has proven that relocation of the kinetoplast is vital to the growth of long epimastigote cells.
Final Transformation Within the Host System
The trypanasoma cell type that is transmitted from the tsetse fly to its mammalian host is metacyclic. Once this happens, an extensive series of chemical signals occurs to change this metacyclic form into the known and pathogenic cell that induces the symptoms of sleeping sickness. When bitten, the microbe is immediately relocated into the bloodstream of the affected host. This is when the metacyclic form transforms into bloodstream trypomastigotes, otherwise known as the slender form of the trypanosoma cell. The cell than enters phase of exponential growth via binary fission. The human environment is very different from that of the fly’s salivary glands. This series of reproduction serves to adjust the microbe to their new living conditions. After reproduction, the cell’s trnasform for the final time into what is called the stumpy vesion. These cells are now stable and cannot divide in daughter cells anymore. They relocate themselves into an array of bodily fluids such as the lymph nodes or the spinal fluid. Once in these locations, the cell’s main goal is to invade the central nervous system (CNS) to bring forth the symptoms we attribute to African Sleeping Sickness. [10]
Entry and Attack
Entry Within the Mammalian Host
The initial contraction of sleeping sickness comes from the tsetse fly. This insect vector shares the trypanosome cell with its host through an unwarranted exchnage. The fly injects its tube like mouth through the skin of the mammal and by the transmission of their saliva, directly inserts the dangerous cells into the host bloodstream. This mechanism is how trypanosoma was initially shared with mammalian species, however this is not the only way that a person can be infected. Because the cell uses the host bloodstream for travel throughout the body, the disease will infect a developing fetus at any point within the host’s pregnancy. As problematic as this microbe is for growing humans, it is even more dangerous to a developing fetus. Trypanosoma cells have also, in some cases, been found to be trnasmitted through sexual contact. However, this portal of entry has yet to be fully discovered. [11]
Attack through the blood and nervous system
The initial attack of T. Brucei occurs within the bloodvessels. The cells begin to push themselves through the capillary beds of the host epithelial cells causing deep lesions. The goal at this point in the life cycle is to relocate within larger, more favorable blood vessels. These would include any blood vessel that leads to larger organ systems such as the spinal cord. After the lesions have persisted for a few days, the lymph nodes of the host begin to drain in attmept to clear the body of infection allowing the microbes to travel through them, infecting the lymphatic system. This is the point of the life cycle that initiates symptoms such as headache, fever, fatigue, etc. These symptoms are caused by the trypanosoma microbes digesting the cells of the lymphatic system. The infection of the lymphatic system causes an identifiable sequelae: The swelling of a lymph node around the trapezius of the host often referred to as “Winterbottom’s sign”.
The ultimate goal of the microbe T. brucei is to reach the brain through a varitey of flowing parts of the body such as the bloodstream and spinal fluid. The cell’s traveling process to inhabit brain matter takes around fifty days. Research done by neuroscience faculty at the Karolinska Institute in Stockholm, Sweden studied mice to understand at what points the trypanosoma cell became fully immersed into the brain tissue of the host. By the twelth day of the study, researchers found the microbe within the blood capallaries of the brain, with only the occasional parasite found living outside of the vessel walls. At day forty-two, the expirementors began to see trypansoma frquently throughout the parechyma. The amount of cells within this location had only increased by day fifty of the study. Once within the parechyma, the cells were most often found within the white matter of the brain, opposed to the cerebral cortex. The white matter is the deeper tissue parts of the brain. This part of the brain is decribed to be primarily composed of the axons of neurons, which function to send electrical signals from one neuron to the next. [12] It only took an additional five days for these parasitic microbes to move from the white matter to the septal nuclei, confined primarily to the brain parechyma. It was noted that an remarkable abundance of the found cells were surrounding very large vessels of these nuclei. [13]
The largest research question surrounding the inoculation of brain tissue with T. brucei has to do with how this large cell can pass the blood brain barrier, otherwise known as the main filtering system between the brain and capillaries. The blood brain barrier (BBB) is extremely selective in what it allows to pass towards the brain. This structure is one within the human body that has the most security, yet continuously is decieved and allows T. brucei to pass leading to fatality. There are a great varity of theories on how this exchange occurs, but one appears to be more likely than the rest. Using a model system, brain microvascluar endothelial cells (BMECs), expirementors found that the passage of the microbe likely has something to do with calcium channels. With the presence of the trypanosome microbe, the BMECs increased greatly in their oscillatory Ca2+ levels. This indicated the possibility that these microbes can alter the integrity of the monolayers within the BBB. These clear increases can be dictated by either a living T. Brucei cell or even by their secretions. These cells depend largely on the presence of cysteine protease in relatioship with the barrier in order to control its permeability. These complexes likely are the structures that recruit these internal calcium ions. The effectiveness of passing by the microbe has been related to the strength of their cysteine proteases. Currently, the signals and responses that these proteases send have yet to be fully clarified. However, they are very important target locations for antibiotic treatment. [14]
Treatment and Prevention
Treatment
Treatment for sleeping sickness is always recommended. Depending on the stage of the disease, it can be more more difficult to treat and require a range of responses. Beyond these challenges, Sleeping Sickness is quite difficult to treat because of the limitation on availble antibiotics and regimen, which are quite toxic and complicated. Only four drugs have been approved for treatment: pentamidine, suramin, eflornithine and melarsoprol. All of these drugs contain a certain level of toxicity. The least harmful of these prescriptions is pentamidine and suramin, which are used to treat beginning or early stages of the disease. Eflornithine is a medication designed to treat those battling the second stage of sleeping sickness. Starting in 2009, a less common drug nifurtimox has been combined with eflornithine to treat secondary stages of the infection. This is the most common treatment across those countries affected by the disease. This combination appears to be the safest and most effective treatment available at the time as well as the simplest regimen to administer. Melasoprol is the only cleared antibiotic for the last stage of sleeping sickness. [15]
Beyond antibiotic medication, sleeping sickness often requires medical survaillence. In the early stages of the disease, the symptoms are as simple as a fever and malaise. When a patient is showing early signs of the infection, they are put under neurological watch in attempts to spare the patient from the brutality of central nervous system invasion by the microbe. The vigilance increases greatly with patients expressing symptoms of the infection at later stages. If the infection has made its way into the central nervous system, medical professionals will often provide airway management, a blood smear, a complete blood cell count, and a spinal tap in search for trypansome cells within the cerebral spinal fluid. [16]
Prevention
Medical research on antibiotic resistance to sleeping sickness is on the rise. There has yet to be a vaccination designed to create immunity against this harmful disease. Because of this disease’s primary transportation being through an insect vector, it is advised to always wear bug repellant and long-sleeved clothing when travelling through a tsetse dense area. Avoiding these known areas provides the greatest protection against the infection. However, when travel through these locations is unavoidable, it is favored to introduce bush clearing methods as well as wild game culling.
In July of 2000, memebers of the Organization for African Unity met to address the problem of sleeping sickness prevention. It was there that the creation of the Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC) was passed. This campaign worked to determine a method for eradicating sleeping sickness entirely. Their decided plan of action was to abolish the tsetse fly entirely which would ultimately exterminate the microbe entirely. The imposed the use of insectiside both in agriculture as well as livestock and animals herds, fly traps, and a method known as sterile insect technique (SIT) which had proved to eradicate the tsetse fly entirely in Zanzibar, Africa. This method is quite expensive and would be, in some locations, impractical to exterminate. This method includes introducing sterile male flies into the environment with intention of them being the primary mates to wild type, female tsetse flies. This mating is completely pointless since the male flies cannot reproduce. This lack of reprodcution creates no future geenrations ultimately eliminating the fly species as a whole.[17][18]
Conclusion
Trypanosoma brucei remians to provide a variety of questions for the medical community today. A great deal of research continues to look into the mechansims in which this disease invades its mammalian host. The mystery of how the microbe infiltrates the blood brain barrier remains as the most popular research topic surround sleeping sickness. However, the research that has been done thus far has provided a successful approach to treatment for its vistims. Unfortunately, the location in which these infectious tsetse flies reside happens to be a part of the world that is in huge medical need. Most cases of sleeping sickness go undetected and undocumented due to a lack of available medicine. The goal for the future is to be able to provide antibiotic and medical assisstance to these people in great need and eventually eradicate this life-threatening disease.
References
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2270819/=PDF Steverding, Dietmar. “The History of African Trypanosomiasis.” Parasites & Vectors, BioMed Central, 2008
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ https://www.cdc.gov/parasites/sleepingsickness/=PDF “Disease.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 29 Aug. 2012, www.cdc.gov/parasites/sleepingsickness/disease.html.
- ↑ https://www.cdc.gov/parasites/sleepingsickness/disease.html=PDF “Disease.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 29 Aug. 2012
- ↑ [http://jcs.biologists.org/content/118/2/283=PDF Matthews, Keith R. “The Developmental Cell Biology of Trypanosoma Brucei.” Journal of Cell Science, The Company of Biologists Ltd, 15 Jan. 2005, jcs.biologists.org/content/118/2/283.
- ↑ https://microbewiki.kenyon.edu/index.php/Trypanosoma=PDF “Disease.” “Trypanosoma.” Trypanosoma - Microbewiki, 7 Aug. 2010
- ↑ http://www.who.int/mediacentre/factsheets/fs259/en/=PDF WHO. “Trypanosomiasis, Human African (Sleeping Sickness).” World Health Organization, World Health Organization, 21 Mar. 2017
- ↑ https://microbewiki.kenyon.edu/index.php/Trypanosome_Life_Cycle=PDF “Trypanosome Life Cycle.” Trypanosome Life Cycle - Microbewiki, 10 Aug. 2010
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/2348830=PDF Hamm, B, et al. “Differentiation of Trypanosoma Brucei Bloodstream Trypomastigotes from Long Slender to Short Stumpy-like Forms in Axenic Culture.” Molecular and Biochemical Parasitology., U.S. National Library of Medicine, Apr. 1990
- ↑ http://www.who.int/mediacentre/factsheets/fs259/en/=PDF WHO. “Trypanosomiasis, Human African (Sleeping Sickness).” World Health Organization, World Health Organization, 21 Mar. 2017
- ↑ https://medlineplus.gov/ency/article/002344.htm=PDF “White Matter of the Brain: MedlinePlus Medical Encyclopedia.” MedlinePlus, U.S. National Library of Medicine
- ↑ https://onlinelibrary.wiley.com/doi/full/10.1046/j.0305-1846.2001.00306.x=PDF Mulenga, C., et al. “Trypanosoma Brucei Brucei Crosses the Blood–Brain Barrier While Tight Junction Proteins Are Preserved in a Rat Chronic Disease Model.” Neuropathology and Applied Neurobiology, Wiley/Blackwell (10.1111), 21 Dec. 2001
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1570376/=PDF Nikolskaia, O. V. “Blood-Brain Barrier Traversal by African Trypanosomes Requires Calcium Signaling Induced by Parasite Cysteine Protease.” Journal of Clinical Investigation, vol. 116, no. 10, 2006
- ↑ http://www.who.int/trypanosomiasis_african/disease/diagnosis/en/=PDF “Symptoms, Diagnosis and Treatment.” World Health Organization, World Health Organization, 5 Aug. 2016
- ↑ https://emedicine.medscape.com/article/228613-treatment=PDF “African Trypanosomiasis Treatment & Management.” African Trypanosomiasis Treatment & Management: Approach Considerations, Pharmacologic Therapy, Prevention, Medscape, 22 Apr. 2018
- ↑ https://en.wikipedia.org/wiki/Sterile_insect_technique=PDF “Sterile Insect Technique.” Wikipedia, Wikimedia Foundation, 10 Apr. 2018
- ↑ https://en.wikipedia.org/wiki/African_trypanosomiasis=PDF “African Trypanosomiasis.” Wikipedia, Wikimedia Foundation, 23 Apr. 2018
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
