Aggregatibacter actinomycetemcomitans: Difference between revisions
| (115 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
Kingdom: ''Bacteria'' <br>Phylum: ''Proteobacteria'' <br>Class: ''Gammaproteobacteria'' <br>Order: ''Pasteurellales'' <br>Family: ''Pasteurellaceae'' <br>Genus: ''Aggregatibacter'' <br>Species: ''Actinomycetemcomitans'' | Kingdom: ''Bacteria'' <br>Phylum: ''Proteobacteria'' <br>Class: ''Gammaproteobacteria'' <br>Order: ''Pasteurellales'' <br>Family: ''Pasteurellaceae'' <br>Genus: ''Aggregatibacter'' <br>Species: ''Actinomycetemcomitans'' <br> [9] | ||
===Species=== | ===Species=== | ||
| Line 11: | Line 11: | ||
====Description==== | ====Description==== | ||
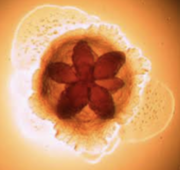
[[File:Screenshot_2024-04-07_at_10.19.28_PM.png|left|180px|thumb|Figure 1. Star-positive ''A.actinomycetemcomitans'' demonstrating its star shaped interior [17]]] ''Aggregatibacter actinomycetemcomitans'' are exogenous, nonmotile, gram-negative bacteria found in the oral cavity [9]. They are capable of growing under both aerobic and anaerobic conditions and are classified as capnophilic, meaning they thrive in high concentrations of carbon dioxide. With an internal, opaque, rought-textured star-shape and translucent colonies, ''A. actinomycetemcomitans'' have three main features: fimbriae, vesicles and extracellular amorphous material [9]. Fimbriae of ''A. actinomycetemcomitans'' are small, filamentous cell surface appendages. They are uniformly distributed in bundles and don’t measure more than two micrometers in diameter. ''A. actinomycetemcomitans'' can either form star-positive, fimbriated colonies with a star shaped interior, or form star-negative, non-fimbriated colonies [9]. Non-fimbriated ''A. actinomycetemcomitans'' are smooth, causing characteristics of poor adherence and poor biofilm forming capacity. The most abundant fimbriae protein in this bacterium is 304-a, a thin protein with a low molecular weight. These fimbriae provide the attachment factor, allowing adhesion to hydroxyapatite, a mineral of the tooth. Vesicles, otherwise known as blebs, of ''A. actinomycetemcomitans'' are lipopolysaccharide in nature, which is common for a great majority of gram-negative bacteria. Highly leukotoxic strains of ''A. actinomycetemcomitans'' tend to have more vesicles. These leukotoxins are described as virulence factors which kill leukocytes allowing them to escape from the host immune system. They play a critical role in the survival of ''A. actinomycetemcomitans'' and periodontal disease progression [14]. ''A. actinomycetemcomitans'' vesicles also contain endotoxin, which is critical in the role of this bacteria as it links cytokine stimulation and pro-inflammatory reactions, leading to the decay of dental pulp and the progression of periodontal disease. Furthermore, vesicles also contain a bacteriocin named ''actinobacillus'', known for adhering to and colonizing the human oral cavity, leading to aggressive periodontitis. Additionally, these vesicles also exhibit adhesive properties and function to deliver toxic materials. Finally, the extracellular amorphous material exhibits bone-resorbing activity and adhesive properties [9]. | |||
<br> | |||
====Significance==== | ====Significance==== | ||
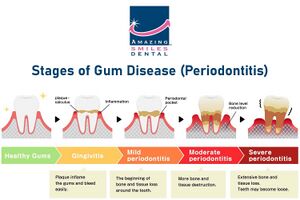
''A. actinomycetemcomitans'' is prevalent in more than one-third of the population [17]. In the United States, approximately 70,000 adolescents develop periodontitis disease annually. Additionally, individuals of African-American descent have a 10 to 15 fold greater risk of developing periodontal disease than Caucasian Americans [15]. There still is not a concrete answer for this significant difference in risk, however several reports state that a contributing factor is the lack of dental care. [[File:Stages-of-Gum-Disease-Periodontitis.jpg|right|300px|thumb|Figure 2. Progression of periodontitis in a patient's tooth [16]]] ''A. actinomycetemcomitans'' is most commonly known as a systemic pathogen, causing the rapid progression of localized aggressive periodontal disease, otherwise known as LAP. ''A. actinomycetemcomitans'' is present in approximately 90% of patients with LAP [2]. Similarly, 97% of this bacteria is commonly found in LJP (localized juvenile periodontitis) lesions [15]. LAP is significant because, as opposed to general periodontitis, it specifically impacts the central incisors and first molars. It is common for LAP patients to experience tooth loss and breakdown of supporting tooth structures when this disease is left untreated [1]. Patients may also experience the risk of swelling of the endocardium, the innermost tissue lining the heart [9]. ''A. actinomycetemcomitans'' thrives in environments of periodontal pockets and blood circulation, being the reason for the prevalence of these risks. This shines light on the importance of maintaining proper oral hygiene because of the significant correlation between oral and cardiovascular health. | |||
==Genome Structure== | ==Genome Structure== | ||
The ''A. actinomycetemcomitans'' genome is made of ranges from 2.0 to 2.7Mb, which is very small compared to other bacteria. It is a single circular chromosome, which contains 2206 genes that make up its genomic structure. These genes include 2129 coding sequenced, along with 19 rRNAs and 54 tRNAs that are need in protein synthesis. Also 4 noncoding RNAs regulate the function that are in the bacteria. Based on differences in its genomic composition and transformation capacity, ''A. actinomycetemcomitans'' can be divided into three evolutionary lineages (I, II, and III) [5]. It has also been shown that serotyping, the common approach to categorization, is not useful for providing a complete description of the species since certain serotypes are not restricted to specific lineages. In addition, ''A. actinomycetemcomitans'' distinct lineages exhibit varying degrees of natural competence. This transformative capacity is linked to horizontal gene transfer and could impact the proportion of a strain's genome that is found within the species. Furthermore, ''A. actinomycetemcomitans's'' prior taxonomic classifications were entirely based on serotyping [5]. | |||
In addition | |||
varying degrees of natural competence. This | |||
linked to horizontal gene transfer and | |||
within the species. | |||
serotyping | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
====Cell Structure and Metabolism==== | ====Cell Structure and Metabolism==== | ||
''Aggretatibacter actinomycetemcomitans'' has a distinct metabolism and cell structure. It has certain characteristic such as it has rod and facultative anaerobic bacteria that is Gram-negative with specific traits [3].The dimensions of its rod-shaped cell normally range from 0.4 to 0.5 µm in width and 1.0 to 1.5 µm in length. These cells sometimes appear as cocci under microscope as seen in Image A of Figure 3 that is collected from clinical sample. This bacterium exhibits environmental flexibility despite not being motile and lacking flagella. It grows poorly in environment air but thrives in 5% CO2. On chocolate agar, colonies are tiny, rough-textured, and firm, sticking to the agar surfaces with great strength as seen in figure B [6]. [[File:Picture1.png|left|250px|thumb|Figure 3. (A) ''A. actinomycetemcomitans'' strain HK1651 on chocolate agar. (B) Distinctive "star shaped" colony incubated and grown in TSBV agar [6]]] ''A. actinomycetemcomitans'' is also unique in that it can create a number of different enzymes and toxins, such as leukotoxin and cytolethal distending toxin (CDT). This adds to its pathogenicity in periodontal infections. Although this bacteria lacks mobility, its rough texture helps it stick to surfaces. This increases colonization and pathogeneticity. ''A. actinomycetemcomitans'' can produce a variety of toxins that are essential to its pathogenicity through metabolism, which can lead to tissue damage and immune evasion in the host [3]. | |||
---- | ---- | ||
====Life Cycle==== | ====Life Cycle==== | ||
The bacterium colonizes the oral mucosa early in life and is inherited by vertical transmission | The bacterium colonizes the oral mucosa early in life and is inherited by vertical transmission. There is a prevalence and gingival localization of ''Aggregatibacter actinomycetemcomitans'' in periodontal lesions of young patients along with intra-tissue bacterial cells and phagocytic cells, which had invaded gingival connective tissue. ''A. actinomycetemcomitans'' utilizes metabolic products from other inhabitants of the biofilm for survival and growth [7]. This bacterium also makes use of lactic acid produced by Streptococcus pyogenes as a nutrient to increase the number of bacteria [4]. More recent studies have demonstrated invasion of ''A. actinomycetemcomitans'' into epithelial cells. Outer membrane protein OmpA1 is associated with the entry of ''A. actinomycetemcomitans'' into gingival epithelial cells caused by the up-regulating F-actin rearrangement via the FAK signaling pathway. Another outer membrane protein, Omp100 promotes adhesion of ''A. actinomycetemcomitans'', and their invasion of gingival epithelial cells. These outer membrane proteins contribute to pathogenicity [4]. Streptococcus pyogenes produce Hydrogen Peroxide, protecting ''A. actinomycetemcomitans'' from oxidative damage and allowing it to migrate deeper into the gingival pocket where they are exposed to the host immune response. Figure 4 shows the invasion of ''A. actinomycetemcomitans'' into epithelial cells. ''A. actinomycetemcomitans'' is now protected from mechanical removal, antibiotics, immune cell phagocytosis, and antibody building. ''A. actinomycetemcomitans'' has developed mechanisms to survive in human serum‐rich environments in vivo, including resistance to complement‐mediated cell lysis and phagocytosis regardless of serotypes and leukotoxin production [8]. [[File:Screenshot_2024-04-24_at_3.38.28_PM.png|right|400px|thumb|Figure 4. Interactions of ''A. actinomycetemcomitans'' in the gingival pocket [8]]]These features allow it to survive in hostile environments of periodontal pockets and blood circulation, where human serum is the predominant nutrient source for bacterial metabolism. In the presence of human serum, bacteriophages are induced to undergo a transition from a lysogenic prophage into the lytic cycle, resulting in bacterial lysis. The pyruvate dehydrogenase complex oxidatively decarboxylates pyruvate to acetyl-coA for the TCA cycle. The TCA cycle, otherwise known as cyclic acid cycle, provides large amounts of energy in aerobic conditions. This energy production and delivery system is required for the replication of phage progeny. Under the PDHc catalytic reaction, bacterial lysis occurs and regulates this bacterium [12]. | ||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
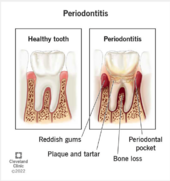
[[File: Screenshot 2024-04-25 at 8.16.11 PM.png|left|170px|thumb|Figure 5. Displays the significant impact of periodontitis on a once healthy tooth [2]]] The pathogenic potential and aggressive nature of ''Aggregatibacter actinomycetemcomitans'' are factors that make it important in human disease. The increased understanding of non-oral infectious involvement in periodontitis is due to the fact that it can cause both oral and systemic infections. It is possible to isolate this using an oral as well as a non-oral range of infectious disorders. Among these are UTIs, bacteremia, endocarditis, osteomyelitis, arthritis, skin infections and abscesses. This periodontitis contributes to an imbalanced formation in periodontal microbiota by these bacteria. As a result, the inflammation destroys the tissues around teeth that connect to them. Its complex role in pathogenesis of many diseases is also demonstrated through its capacity to elude host immune system and interact with other microbial species found in oral cavity [11]. Also, ''A. actinomycetemcomitans'' may be connected with some virulence factors including leukotoxin as a potential one which can lead to apoptosis or programmed cell death among epithelial cells resulting into tissue damage hence localized aggressive periodontitis. Moreover, ''A.actinomycetemcomitans'' produces CDTs known as cytolethal distending toxins that affect DNA via interaction with the host cell cycle. Clinical signs of ''A. actinomycetemcomitans'' infections include bone loss leading to pocket formations around the tooth root [1]. | |||
==Treating ''A.actinomycetemcomitans''== | |||
Ways to maintain periodontitis and treat ''Aggregatibacter actinomycetemcomitans'' include utilizing deep cleaning methods (scaling and root planing), blue light, amoxicillin-metronidazole treatment, and systemic ciprofloxacin treatment [2][14]. [[File:Jcm-08-01079-g001.png|200px|thumb|right|Figure 6. Mechanical and chemical ways of treatment and prevention of ''A.actinomycetemcomitans'' [8]]] | |||
== | ==References== | ||
1 | [1] Belibasakis, G. N., Maula, T., Bao, K., Lindholm, M., Bostanci, N., Oscarsson, J., Ihalin, R., & Johansson, A.(2019). Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens(Basel, Switzerland), 8(4), 222. | ||
2 | [2] C. C. Medical. (n.d.). Periodontitis (gum disease): Symptoms, stages & treatment. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/16620-periodontitis | ||
3 | [3] Kelk, P., Abd, H., Claesson, R., SandstrÃm, G., SjÃstedt, A., & Johansson, A. (2011). Cellular and molecular response of human macrophages exposed to | ||
:: Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death & Disease, 2(3), e126–e126. | :: Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death & Disease, 2(3), e126–e126. | ||
4 | [4] Lindholm, M., Min Aung, K., Nyunt Wai, S., & Oscarsson, J. (2019). Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter | ||
:: aphrophilus serum resistance. Journal of Oral Microbiology, 11(1), 1536192. | :: aphrophilus serum resistance. Journal of Oral Microbiology, 11(1), 1536192. | ||
5 | [5] May, A. C., Ehrlich, R. L., Balashov, S., Ehrlich, G. D., Shanmugam, M., Fine, D. H., ... & Cugini, C. (2016).Complete genome sequence of Aggregatibacter | ||
:: actinomycetemcomitans strain IDH781. Genome Announcements, 4(6), 10-1128. | :: actinomycetemcomitans strain IDH781. Genome Announcements, 4(6), 10-1128. | ||
[6] Nørskov-Lauritsen, N., Claesson, R., Jensen, A. B., Åberg, C. H., & Haubek, D. (2019). Aggregatibacter Actinomycetemcomitans: Clinical Significance of a | |||
:: Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens, 8(4). | :: Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens, 8(4). | ||
[7] Oscarsson, J., Claesson, R., Lindholm, M., Höglund Åberg, C., & Johansson, A. (2019). Tools of Aggregatibacter actinomycetemcomitans to evade the host response. :: Journal of clinical medicine, 8(7), 1079. | |||
[8] Ozuna, H., Snider, I., Belibasakis, G. N., Oscarsson, J., Johansson, A., & Uriarte, S. M. (2022). Aggregatibacter actinomycetemcomitans and Filifactor alocis Two exotoxin-producing oral pathogens. Frontiers in oral health, | |||
:: 3, 981343. | :: 3, 981343. | ||
[9] Raja, M., Ummer, F., & Dhivakar, C. P. (2014). Aggregatibacter actinomycetemcomitans–a tooth killer?. Journal of clinical and diagnostic research: JCDR, 8(8), ZE13. | |||
[10] Sampathkumar, V., Velusamy, S. K., Godboley, D., & Fine, D. H. (2017). Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter | |||
:: actinomycetemcomitans. Scientific reports, 7(1), 1887. | :: actinomycetemcomitans. Scientific reports, 7(1), 1887. | ||
12 | [11] Socransky, S. S., & Haffajee, A. D. (2002). Dental biofilms: difficult therapeutic targets. Periodontology 2000, 28(1), 12–55. | ||
[12] Tang-Siegel, G. G. (2023). Human Serum Mediated Bacteriophage Life Cycle Switch in A2gregatibacter actinomycetemcomitans Is Linked to Pyruvate Dehydrogenase | |||
:: Complex. Life, 13(2), 436. | :: Complex. Life, 13(2), 436. | ||
13 | [13] Tsai, C. C., Ho, Y. P., Chou, Y. S., Ho, K. Y., Wu, Y. M., & Lin, Y. C. (2018). Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human | ||
:: periodontitis–A historic review with emphasis on JP2. The Kaohsiung journal of medical sciences, 34(4), 186-193. | :: periodontitis–A historic review with emphasis on JP2. The Kaohsiung journal of medical sciences, 34(4), 186-193. | ||
14 | [14] Vega, B. A., Belinka Jr, B. A., & Kachlany, S. C. (2019). Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera®): mechanisms of action and | ||
:: therapeutic applications. Toxins, 11(9), 489. | :: therapeutic applications. Toxins, 11(9), 489. | ||
15 | [15] Yoshida, A., Bouziane, A., Erraji, S., Lakhdar, L., Rhissassi, M., Miyazaki, H., ... & Ennibi, O. (2021). Etiology of aggressive periodontitis in individuals of African descent. Japanese Dental Science Review, 57, 20-26. | ||
[16] Periodontitis - gum disease: Symptoms, causes, & treatment. Amazing Smiles. (2023, August 22). https://amazingsmiles.com.au/periodontitis-symptoms-and-treatment-options/ | |||
[17] Unique exotoxin-producing oral bacterium. (n.d.). https://schaechter.asmblog.org/schaechter/2013/07/aggregatibacter-actinomycetemcomitans-a-unique-exotoxin-producing-oral-bacterium-.html | |||
==Authors== | |||
Page authored by Hannah Pedersen and Nirali Patel, students of Professor Jay Lennon at Indiana University. | |||
Latest revision as of 14:21, 26 April 2024
Classification
Kingdom: Bacteria
Phylum: Proteobacteria
Class: Gammaproteobacteria
Order: Pasteurellales
Family: Pasteurellaceae
Genus: Aggregatibacter
Species: Actinomycetemcomitans
[9]
Species
NCBI: HK1651 Taxonomy
Aggregatibacter Actinomycetemcomitans
Description and Significance
Description
Aggregatibacter actinomycetemcomitans are exogenous, nonmotile, gram-negative bacteria found in the oral cavity [9]. They are capable of growing under both aerobic and anaerobic conditions and are classified as capnophilic, meaning they thrive in high concentrations of carbon dioxide. With an internal, opaque, rought-textured star-shape and translucent colonies, A. actinomycetemcomitans have three main features: fimbriae, vesicles and extracellular amorphous material [9]. Fimbriae of A. actinomycetemcomitans are small, filamentous cell surface appendages. They are uniformly distributed in bundles and don’t measure more than two micrometers in diameter. A. actinomycetemcomitans can either form star-positive, fimbriated colonies with a star shaped interior, or form star-negative, non-fimbriated colonies [9]. Non-fimbriated A. actinomycetemcomitans are smooth, causing characteristics of poor adherence and poor biofilm forming capacity. The most abundant fimbriae protein in this bacterium is 304-a, a thin protein with a low molecular weight. These fimbriae provide the attachment factor, allowing adhesion to hydroxyapatite, a mineral of the tooth. Vesicles, otherwise known as blebs, of A. actinomycetemcomitans are lipopolysaccharide in nature, which is common for a great majority of gram-negative bacteria. Highly leukotoxic strains of A. actinomycetemcomitans tend to have more vesicles. These leukotoxins are described as virulence factors which kill leukocytes allowing them to escape from the host immune system. They play a critical role in the survival of A. actinomycetemcomitans and periodontal disease progression [14]. A. actinomycetemcomitans vesicles also contain endotoxin, which is critical in the role of this bacteria as it links cytokine stimulation and pro-inflammatory reactions, leading to the decay of dental pulp and the progression of periodontal disease. Furthermore, vesicles also contain a bacteriocin named actinobacillus, known for adhering to and colonizing the human oral cavity, leading to aggressive periodontitis. Additionally, these vesicles also exhibit adhesive properties and function to deliver toxic materials. Finally, the extracellular amorphous material exhibits bone-resorbing activity and adhesive properties [9].
Significance
A. actinomycetemcomitans is prevalent in more than one-third of the population [17]. In the United States, approximately 70,000 adolescents develop periodontitis disease annually. Additionally, individuals of African-American descent have a 10 to 15 fold greater risk of developing periodontal disease than Caucasian Americans [15]. There still is not a concrete answer for this significant difference in risk, however several reports state that a contributing factor is the lack of dental care.
A. actinomycetemcomitans is most commonly known as a systemic pathogen, causing the rapid progression of localized aggressive periodontal disease, otherwise known as LAP. A. actinomycetemcomitans is present in approximately 90% of patients with LAP [2]. Similarly, 97% of this bacteria is commonly found in LJP (localized juvenile periodontitis) lesions [15]. LAP is significant because, as opposed to general periodontitis, it specifically impacts the central incisors and first molars. It is common for LAP patients to experience tooth loss and breakdown of supporting tooth structures when this disease is left untreated [1]. Patients may also experience the risk of swelling of the endocardium, the innermost tissue lining the heart [9]. A. actinomycetemcomitans thrives in environments of periodontal pockets and blood circulation, being the reason for the prevalence of these risks. This shines light on the importance of maintaining proper oral hygiene because of the significant correlation between oral and cardiovascular health.
Genome Structure
The A. actinomycetemcomitans genome is made of ranges from 2.0 to 2.7Mb, which is very small compared to other bacteria. It is a single circular chromosome, which contains 2206 genes that make up its genomic structure. These genes include 2129 coding sequenced, along with 19 rRNAs and 54 tRNAs that are need in protein synthesis. Also 4 noncoding RNAs regulate the function that are in the bacteria. Based on differences in its genomic composition and transformation capacity, A. actinomycetemcomitans can be divided into three evolutionary lineages (I, II, and III) [5]. It has also been shown that serotyping, the common approach to categorization, is not useful for providing a complete description of the species since certain serotypes are not restricted to specific lineages. In addition, A. actinomycetemcomitans distinct lineages exhibit varying degrees of natural competence. This transformative capacity is linked to horizontal gene transfer and could impact the proportion of a strain's genome that is found within the species. Furthermore, A. actinomycetemcomitans's prior taxonomic classifications were entirely based on serotyping [5].
Cell Structure, Metabolism and Life Cycle
Cell Structure and Metabolism
Aggretatibacter actinomycetemcomitans has a distinct metabolism and cell structure. It has certain characteristic such as it has rod and facultative anaerobic bacteria that is Gram-negative with specific traits [3].The dimensions of its rod-shaped cell normally range from 0.4 to 0.5 µm in width and 1.0 to 1.5 µm in length. These cells sometimes appear as cocci under microscope as seen in Image A of Figure 3 that is collected from clinical sample. This bacterium exhibits environmental flexibility despite not being motile and lacking flagella. It grows poorly in environment air but thrives in 5% CO2. On chocolate agar, colonies are tiny, rough-textured, and firm, sticking to the agar surfaces with great strength as seen in figure B [6].
A. actinomycetemcomitans is also unique in that it can create a number of different enzymes and toxins, such as leukotoxin and cytolethal distending toxin (CDT). This adds to its pathogenicity in periodontal infections. Although this bacteria lacks mobility, its rough texture helps it stick to surfaces. This increases colonization and pathogeneticity. A. actinomycetemcomitans can produce a variety of toxins that are essential to its pathogenicity through metabolism, which can lead to tissue damage and immune evasion in the host [3].
Life Cycle
The bacterium colonizes the oral mucosa early in life and is inherited by vertical transmission. There is a prevalence and gingival localization of Aggregatibacter actinomycetemcomitans in periodontal lesions of young patients along with intra-tissue bacterial cells and phagocytic cells, which had invaded gingival connective tissue. A. actinomycetemcomitans utilizes metabolic products from other inhabitants of the biofilm for survival and growth [7]. This bacterium also makes use of lactic acid produced by Streptococcus pyogenes as a nutrient to increase the number of bacteria [4]. More recent studies have demonstrated invasion of A. actinomycetemcomitans into epithelial cells. Outer membrane protein OmpA1 is associated with the entry of A. actinomycetemcomitans into gingival epithelial cells caused by the up-regulating F-actin rearrangement via the FAK signaling pathway. Another outer membrane protein, Omp100 promotes adhesion of A. actinomycetemcomitans, and their invasion of gingival epithelial cells. These outer membrane proteins contribute to pathogenicity [4]. Streptococcus pyogenes produce Hydrogen Peroxide, protecting A. actinomycetemcomitans from oxidative damage and allowing it to migrate deeper into the gingival pocket where they are exposed to the host immune response. Figure 4 shows the invasion of A. actinomycetemcomitans into epithelial cells. A. actinomycetemcomitans is now protected from mechanical removal, antibiotics, immune cell phagocytosis, and antibody building. A. actinomycetemcomitans has developed mechanisms to survive in human serum‐rich environments in vivo, including resistance to complement‐mediated cell lysis and phagocytosis regardless of serotypes and leukotoxin production [8].
These features allow it to survive in hostile environments of periodontal pockets and blood circulation, where human serum is the predominant nutrient source for bacterial metabolism. In the presence of human serum, bacteriophages are induced to undergo a transition from a lysogenic prophage into the lytic cycle, resulting in bacterial lysis. The pyruvate dehydrogenase complex oxidatively decarboxylates pyruvate to acetyl-coA for the TCA cycle. The TCA cycle, otherwise known as cyclic acid cycle, provides large amounts of energy in aerobic conditions. This energy production and delivery system is required for the replication of phage progeny. Under the PDHc catalytic reaction, bacterial lysis occurs and regulates this bacterium [12].
Ecology and Pathogenesis
The pathogenic potential and aggressive nature of Aggregatibacter actinomycetemcomitans are factors that make it important in human disease. The increased understanding of non-oral infectious involvement in periodontitis is due to the fact that it can cause both oral and systemic infections. It is possible to isolate this using an oral as well as a non-oral range of infectious disorders. Among these are UTIs, bacteremia, endocarditis, osteomyelitis, arthritis, skin infections and abscesses. This periodontitis contributes to an imbalanced formation in periodontal microbiota by these bacteria. As a result, the inflammation destroys the tissues around teeth that connect to them. Its complex role in pathogenesis of many diseases is also demonstrated through its capacity to elude host immune system and interact with other microbial species found in oral cavity [11]. Also, A. actinomycetemcomitans may be connected with some virulence factors including leukotoxin as a potential one which can lead to apoptosis or programmed cell death among epithelial cells resulting into tissue damage hence localized aggressive periodontitis. Moreover, A.actinomycetemcomitans produces CDTs known as cytolethal distending toxins that affect DNA via interaction with the host cell cycle. Clinical signs of A. actinomycetemcomitans infections include bone loss leading to pocket formations around the tooth root [1].
Treating A.actinomycetemcomitans
Ways to maintain periodontitis and treat Aggregatibacter actinomycetemcomitans include utilizing deep cleaning methods (scaling and root planing), blue light, amoxicillin-metronidazole treatment, and systemic ciprofloxacin treatment [2][14].
References
[1] Belibasakis, G. N., Maula, T., Bao, K., Lindholm, M., Bostanci, N., Oscarsson, J., Ihalin, R., & Johansson, A.(2019). Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens(Basel, Switzerland), 8(4), 222.
[2] C. C. Medical. (n.d.). Periodontitis (gum disease): Symptoms, stages & treatment. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/16620-periodontitis
[3] Kelk, P., Abd, H., Claesson, R., SandstrÃm, G., SjÃstedt, A., & Johansson, A. (2011). Cellular and molecular response of human macrophages exposed to
- Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death & Disease, 2(3), e126–e126.
[4] Lindholm, M., Min Aung, K., Nyunt Wai, S., & Oscarsson, J. (2019). Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter
- aphrophilus serum resistance. Journal of Oral Microbiology, 11(1), 1536192.
[5] May, A. C., Ehrlich, R. L., Balashov, S., Ehrlich, G. D., Shanmugam, M., Fine, D. H., ... & Cugini, C. (2016).Complete genome sequence of Aggregatibacter
- actinomycetemcomitans strain IDH781. Genome Announcements, 4(6), 10-1128.
[6] Nørskov-Lauritsen, N., Claesson, R., Jensen, A. B., Åberg, C. H., & Haubek, D. (2019). Aggregatibacter Actinomycetemcomitans: Clinical Significance of a
- Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens, 8(4).
[7] Oscarsson, J., Claesson, R., Lindholm, M., Höglund Åberg, C., & Johansson, A. (2019). Tools of Aggregatibacter actinomycetemcomitans to evade the host response. :: Journal of clinical medicine, 8(7), 1079.
[8] Ozuna, H., Snider, I., Belibasakis, G. N., Oscarsson, J., Johansson, A., & Uriarte, S. M. (2022). Aggregatibacter actinomycetemcomitans and Filifactor alocis Two exotoxin-producing oral pathogens. Frontiers in oral health,
- 3, 981343.
[9] Raja, M., Ummer, F., & Dhivakar, C. P. (2014). Aggregatibacter actinomycetemcomitans–a tooth killer?. Journal of clinical and diagnostic research: JCDR, 8(8), ZE13.
[10] Sampathkumar, V., Velusamy, S. K., Godboley, D., & Fine, D. H. (2017). Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter
- actinomycetemcomitans. Scientific reports, 7(1), 1887.
[11] Socransky, S. S., & Haffajee, A. D. (2002). Dental biofilms: difficult therapeutic targets. Periodontology 2000, 28(1), 12–55.
[12] Tang-Siegel, G. G. (2023). Human Serum Mediated Bacteriophage Life Cycle Switch in A2gregatibacter actinomycetemcomitans Is Linked to Pyruvate Dehydrogenase
- Complex. Life, 13(2), 436.
[13] Tsai, C. C., Ho, Y. P., Chou, Y. S., Ho, K. Y., Wu, Y. M., & Lin, Y. C. (2018). Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human
- periodontitis–A historic review with emphasis on JP2. The Kaohsiung journal of medical sciences, 34(4), 186-193.
[14] Vega, B. A., Belinka Jr, B. A., & Kachlany, S. C. (2019). Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera®): mechanisms of action and
- therapeutic applications. Toxins, 11(9), 489.
[15] Yoshida, A., Bouziane, A., Erraji, S., Lakhdar, L., Rhissassi, M., Miyazaki, H., ... & Ennibi, O. (2021). Etiology of aggressive periodontitis in individuals of African descent. Japanese Dental Science Review, 57, 20-26.
[16] Periodontitis - gum disease: Symptoms, causes, & treatment. Amazing Smiles. (2023, August 22). https://amazingsmiles.com.au/periodontitis-symptoms-and-treatment-options/
[17] Unique exotoxin-producing oral bacterium. (n.d.). https://schaechter.asmblog.org/schaechter/2013/07/aggregatibacter-actinomycetemcomitans-a-unique-exotoxin-producing-oral-bacterium-.html
Authors
Page authored by Hannah Pedersen and Nirali Patel, students of Professor Jay Lennon at Indiana University.