Aggregatibacter actinomycetemcomitans: Difference between revisions
| Line 30: | Line 30: | ||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
''Aggregatibacter actinomycetemcomitans'' | The pathogenic potential and aggressive nature of ''Aggregatibacter actinomycetemcomitans'' are factors that make it important in human disease. The increased understanding of non-oral infectious involvement in periodontitis is due to the fact that it can cause both oral and systemic infections. It is possible to isolate this using an oral as well as a non-oral range of infectious disorders. Among these are UTIs, bacteremia, endocarditis, osteomyelitis, arthritis, skin infections and abscesses. This periodontitis contributes to an imbalanced formation in periodontal microbiota by these bacteria. As a result, the inflammation destroys the tissues around teeth that connect to them. Its complex role in pathogenesis of many diseases is also demonstrated through its capacity to elude host immune system and interact with other microbial species found in oral cavity (Socransky et al., 2002). Also, ''A. actinomycetemcomitans'' may be connected with some virulence factors including leukotoxin as a potential one which can lead to apoptosis or programmed cell death among epithelial cells resulting into tissue damage hence localized aggressive periodontitis. Moreover, ''A.actinomycetemcomitans'' produces CDTs known as cytolethal distending toxins that affect DNA via interaction with the host cell cycle. Clinical signs of ''A. actinomycetemcomitans'' infections include bone loss leading to pocket formations around the tooth root. | ||
oral cavity | |||
virulence factors | |||
==Reference== | ==Reference== | ||
Revision as of 12:32, 25 April 2024
Classification
Kingdom: Bacteria
Phylum: Proteobacteria
Class: Gammaproteobacteria
Order: Pasteurellales
Family: Pasteurellaceae
Genus: Aggregatibacter
Species: Actinomycetemcomitans
Species
NCBI: HK1651 Taxonomy
Aggregatibacter Actinomycetemcomitans
Description and Significance
Description
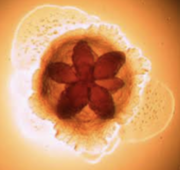
In 1975, scientists Killian and Schiott were the first to demonstrate that Aggregatibacter actinomycetemcomitans was present in dental plaque and from that point on, more and more important discoveries about A. actinomycetemcomitans have been made. Aggregatibacter actinomycetemcomitans are nonmotile, gram-negative bacteria capable of growing under both aerobic and anaerobic conditions. They also thrive in high concentrations of carbon dioxide. What’s really interesting about this bacterium is its shape. You can see it has an internal star-shape and translucent colonies. Aggregatibacter actinomycetemcomitans has three main features including fimbriae, vesicles and extracellular amorphous materials. Fimbriae of A. actinomycetemcomitans are small, filamentous cell surface appendages. They are uniformly distributed in bundles and don’t measure more than two micrometers in diameter. A. actinomycetemcomitans can either have star-positive fimbriated colonies or have a non-fimbriated strain. Non-fimbriated A. actinomycetemcomitans are smooth, leading to poor adherence and poor biofilm forming capacity. The most abundant fimbriae protein is 304-a, a thin protein with a low molecular weight. These fimbriae provide the attachment factor, allowing adhesion. Vesicles, otherwise known as blebs, of A. actinomycetemcomitans are lipopolysaccharide in nature, which is common for a great majority of gram-negative bacteria. Highly leukotoxic strains of A. actinomycetemcomitans tend to have more vesicles. These leukotoxins are described as virulence factors that kill leukocytes allowing them to escape from the host immune system. They play a critical role in the survival of A. actinomycetemcomitans and periodontal disease progression. A. actinomycetemcomitans vesicles also contain endotoxin, which is critical in the role of A. actinomycetemcomitans. Endotoxin basically links cytokine stimulation and proinflammatory reactions, leading to the decay of dental pulp and periodontal disease. Vesicles also contain a bacteriocin named actinobacillus which is known for adhering to and colonizing the human oral cavity, leading to aggressive periodontitis. Furthermore, these vesicles also exhibit adhesive properties and function to deliver toxic materials. Finally, the extracellular amorphous material exhibits bone-resorbing activity and adhesive properties as well.
Significance
A. actinomycetemcomitans inhabits the mouths of one-third or more of the population.
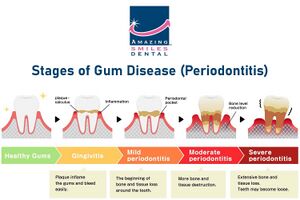
It is most commonly known as a systemic pathogen, causing the rapid progression of localized aggressive periodontal disease, otherwise known as LAP. As a matter of fact, A. actinomycetemcomitans is present in approximately 90% of patients with LAP. LAP is significant because, as opposed to general periodontitis, it specifically impacts the central incisors (which are your top two teeth and bottom two teeth) and first molars. It is common for LAP patients to have tooth loss and breakdown of supporting tooth structures when this disease is left untreated. Another risk is through blood circulation, there is the risk of swelling of the endocardium, the innermost tissue lining the heart. This actually makes a lot of sense because A. actinomycetemcomitans thrives in environments of periodontal pockets and blood circulation. This shines light on the importance of maintaining proper oral hygiene because of the significant correlation between oral and cardiovascular health. Referring to the general population, approximately 70,000 adolescents in the United States develop periodontitis disease annually. And individuals of African-American descent have a 10 to 15 fold greater risk of developing periodontal disease than Caucasian Americans.
Genome Structure
The A. actinomycetemcomitans genome is made of ranges from 2.0 to 2.7Mb, which is very small compared to other bacteria. It is a single circular chromosome, which contains 2206 genes that make up its genomic structure. These genes include 2129 coding sequenced, along with 19 rRNAs and 54 tRNAs that are need in protein synthesis. Also 4 noncoding RNAs regulate the function that are in the bacteria. Based on differences in its genomic composition and transformation capacity, A. actinomycetemcomitans can be divided into three evolutionary lineages (I, II, and III). It has also been shown that serotyping, the common approach to categorization, is not useful for providing a complete description of the species since certain serotypes are not restricted to specific lineages. In addition, A. actinomycetemcomitans distinct lineages exhibit varying degrees of natural competence. This transformative capacity is linked to horizontal gene transfer and could impact the proportion of a strain's genome that is found within the species. Furthermore, A. actinomycetemcomitans's prior taxonomic classifications were entirely based on serotyping.
Cell Structure, Metabolism and Life Cycle
Cell Structure and Metabolism
Aggretatibacter actinomycetemcomitans has a distinct metabolism and cell structure. It has certain characteristic such as it has rod and facultative anaerobic bacteria that is Gram-negative with specific traits.The dimensions of its rod-shaped cell normally range from 0.4 to 0.5 µm in width and 1.0 to 1.5 µm in length. These cells sometimes appear as cocci under microscope as seen in figure that is collected from clinical smaple. This bacterium exhibits environmental flexibility despite not being motile and lacking flagella. It grows poorly in environment air but thrives in 5% CO2. On chocolate agar, colonies are tiny, rough-textured, and firm, sticking to the agar surfaces with great strength. A. actinomycetemcomitans is also unique in that it can create a number of different enzymes and toxins, such as leukotoxin and cytolethal distending toxin (CDT). This add to its pathogenicity in periodontal infections. Although this bacteria lacks mobility, its rough texture helps it stick to surfaces. This increases colonization and pathogeneticity. A. actinomycetemcomitans can produce a variety of toxins that are essential to its pathogenicity through metabolism, which can lead to tissue damage and immune evasion in the host.
Life Cycle
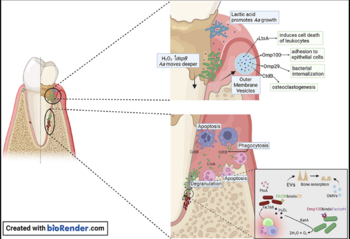
The bacterium colonizes the oral mucosa early in life and is inherited by vertical transmission from close relatives. There is a prevalence and gingival localization of Aggregatibacter actinomycetemcomitans in periodontal lesions of young patients along with intra-tissue bacterial cells and phagocytic cells, which had invaded gingival connective tissue. A. actinomycetemcomitans utilizes metabolic products from other inhabitants of the biofilm for survival and growth. This bacterium also makes use of lactic acid produced by Streptococcus pyogenes as a nutrient to increase its numbers. More recent studies have demonstrated invasion of A. actinomycetemcomitans into epithelial cells. Outer membrane protein OmpA1 is associated with the entry of A. actinomycetemcomitans into gingival epithelial cells caused by the up-regulating F-actin rearrangement via the FAK signaling pathway. Another outer membrane protein, Omp100 promotes adhesion of A. actinomycetemcomitans, and their invasion of gingival epithelial cells. These outer membrane proteins contribute to pathogenicity which will be discussed later on. Streptococcus pyogenes produce Hydrogen peroxide, protecting A. actinomycetemcomitans from oxidative damage and allowing it to migrate deeper into the gingival pocket where they are exposed to th host immune response. The image on the top right shows the invasion of A. actinomycetemcomitans into epithelial cells. A. actinomycetemcomitans is now protected from mechanical removal, antibiotics, immune cell phagocytosis, and antibody building. A. actinomycetemcomitans has developed mechanisms to survive in human serum‐rich environments in vivo, including resistance to complement‐mediated cell lysis and phagocytosis regardless of serotypes and leukotoxin production.
These features allow it to survive in hostile environments of periodontal pockets and blood circulation, where human serum is the predominant nutrient source for bacterial metabolism. Human serum is basically blood plasma without clotting factors. In the presence of human serum, bacteriophages are induced to undergo a transition from a lysogenic prophage into the lytic cycle, resulting in bacterial lysis. The pyruvate dehydrogenase complex oxidatively decarboxylates pyruvate to acetyl-coA for the TCA cycle. The TCA cycle, otherwise known as cyclic acid cycle, provides large amounts of energy in aerobic conditions. This energy production and delivery system is required for the replication of phage progeny. Under the PDHc catalytic reaction, bacterial lysis occurs and regulates this bacterium.
Ecology and Pathogenesis
The pathogenic potential and aggressive nature of Aggregatibacter actinomycetemcomitans are factors that make it important in human disease. The increased understanding of non-oral infectious involvement in periodontitis is due to the fact that it can cause both oral and systemic infections. It is possible to isolate this using an oral as well as a non-oral range of infectious disorders. Among these are UTIs, bacteremia, endocarditis, osteomyelitis, arthritis, skin infections and abscesses. This periodontitis contributes to an imbalanced formation in periodontal microbiota by these bacteria. As a result, the inflammation destroys the tissues around teeth that connect to them. Its complex role in pathogenesis of many diseases is also demonstrated through its capacity to elude host immune system and interact with other microbial species found in oral cavity (Socransky et al., 2002). Also, A. actinomycetemcomitans may be connected with some virulence factors including leukotoxin as a potential one which can lead to apoptosis or programmed cell death among epithelial cells resulting into tissue damage hence localized aggressive periodontitis. Moreover, A.actinomycetemcomitans produces CDTs known as cytolethal distending toxins that affect DNA via interaction with the host cell cycle. Clinical signs of A. actinomycetemcomitans infections include bone loss leading to pocket formations around the tooth root.
Reference
1) Belibasakis, G. N., Maula, T., Bao, K., Lindholm, M., Bostanci, N., Oscarsson, J., Ihalin, R., & Johansson, A.(2019). Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens (Basel,
- Switzerland), 8(4), 222.
2) Flemmig, T. F. (1999). Periodontitis. Annals of periodontology, 4(1), 32-37.
3) Kelk, P., Abd, H., Claesson, R., SandstrÃm, G., SjÃstedt, A., & Johansson, A. (2011). Cellular and molecular response of human macrophages exposed to
- Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death & Disease, 2(3), e126–e126.
4) Lindholm, M., Min Aung, K., Nyunt Wai, S., & Oscarsson, J. (2019). Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter
- aphrophilus serum resistance. Journal of Oral Microbiology, 11(1), 1536192.
5) May, A. C., Ehrlich, R. L., Balashov, S., Ehrlich, G. D., Shanmugam, M., Fine, D. H., ... & Cugini, C. (2016).Complete genome sequence of Aggregatibacter
- actinomycetemcomitans strain IDH781. Genome Announcements, 4(6), 10-1128.
6) Nedergaard, S., Kobel, C. M., Nielsen, M. B., Møller, R. T., Jensen, A. B., & Nørskov-Lauritsen, N. (2019).
- Whole Genome Sequencing of Aggregatibacter actinomycetemcomitans Cultured from Blood Stream Infections Reveals Three Major Phylogenetic Groups Including a
- Novel Lineage Expressing Serotype a Membrane O Polysaccharide. Pathogens, 8(4), 256.
7) Nørskov-Lauritsen, N., Claesson, R., Jensen, A. B., Åberg, C. H., & Haubek, D. (2019). Aggregatibacter Actinomycetemcomitans: Clinical Significance of a
- Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens, 8(4).
8) Oscarsson, J., Claesson, R., Lindholm, M., Höglund Åberg, C., & Johansson, A. (2019). Tools of Aggregatibacter actinomycetemcomitans to evade the host response. :: Journal of clinical medicine, 8(7), 1079.
9) Ozuna, H., Snider, I., Belibasakis, G. N., Oscarsson, J., Johansson, A., & Uriarte, S. M. (2022). Aggregatibacter actinomycetemcomitans and Filifactor alocis Two exotoxin-producing oral pathogens. Frontiers in oral health,
- 3, 981343.
10) Raja, M., Ummer, F., & Dhivakar, C. P. (2014). Aggregatibacter actinomycetemcomitans–a tooth killer?. Journal of clinical and diagnostic research: JCDR, 8(8), ZE13.
11) Sampathkumar, V., Velusamy, S. K., Godboley, D., & Fine, D. H. (2017). Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter
- actinomycetemcomitans. Scientific reports, 7(1), 1887.
12) Tang-Siegel, G. G. (2023). Human Serum Mediated Bacteriophage Life Cycle Switch in Aggregatibacter actinomycetemcomitans Is Linked to Pyruvate Dehydrogenase
- Complex. Life, 13(2), 436.
13) Tsai, C. C., Ho, Y. P., Chou, Y. S., Ho, K. Y., Wu, Y. M., & Lin, Y. C. (2018). Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human
- periodontitis–A historic review with emphasis on JP2. The Kaohsiung journal of medical sciences, 34(4), 186-193.
14) Vega, B. A., Belinka Jr, B. A., & Kachlany, S. C. (2019). Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera®): mechanisms of action and
- therapeutic applications. Toxins, 11(9), 489.
15) Yoshida, A., Bouziane, A., Erraji, S., Lakhdar, L., Rhissassi, M., Miyazaki, H., ... & Ennibi, O. (2021). Etiology of aggressive periodontitis in individuals of African descent. Japanese Dental Science Review, 57, 20-26.
Authors
Page authored by Hannah Pedersen and Nirali Patel, students of Professor Jay Lennon at Indiana University.