Aliivibrio Fischeri and the Role of Quorum Sensing
Introduction
By Elizabeth Matteri
Aliivibrio fischeri are gram-negative Proteobacteria that are capable of producing bioluminescence. Although previously termed Vibrio fischeri, researchers proposed a reclassification of the species and changed the name in 2007 (Urbanczyk et al. 2007). The bacteria, while found throughout the ocean, colonize the light organs of specific kinds of squid and fish species. They can also be found on decaying matter in the ocean (Schaefer et al. 1996, Boettcher et al. 1990).
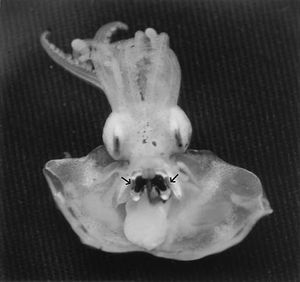
The most understood relationship that Aliivibrio fischeri forms is with the Hawaiian Bobtail squid (Euprymna scolopes). This squid is usually found near the shore, is nocturnal, and has two bilobed light organs on its ventral side (Wei et al. 1989). These light organs are made up of sacks, or crypts, that hold the Aliivibrio fischeri as well as two lenses and a reflector (Wei et al. 1989). This symbiotic relationship is a key predator avoidance strategy used by the squid, as the light that the bacteria emit is used to counterilluminate the squid against moonlight or starlight shining into the ocean, making them less visible to predators. In other words, the ventral side of the animal is brightened and appears more similar in color to the lighter water nearer to the surface, so a predator below the squid is less likely to notice its presence due to camouflage (Jones et al. 2004). Despite the symbiosis, it is important to note that both the bacteria and the Hawaiian Bobtail squid are capable of surviving independently of each other (Lupp et al. 2003).
Due to the many years of research that have focused on this symbiotic relationship, Aliivibrio fischeri and the Hawaiian Bobtail squid are model organisms for the ways in which bacteria can colonize a host and influence the development of a host (Visick et al. 2006). For many years, the way in which the bacteria would colonize the light organs of the squid was very poorly understood, but more recent studies are beginning to bring this process to light (Visick et al. 2006). Also, because of how the bacteria use quorum sensing as an integral part of their colonization of the host squid, Aliivibrio fischeri are a model organism used to better understand the role of quorum sensing in general, and they will hopefully lead to the development of quorum sensing inhibitor drugs in the future that can help prevent pathogenesis by other bacterial species (Visick et al. 2006). Not only this, but a quorum sensing inhibitory drug could provide an alternative form of treatment to antibiotics, as many human pathogens become drug resistant—which would help save many lives (Suga et al. 2003).


Quorum Sensing and Bioluminescence
Quorum sensing is a type of signaling pathway that is used by gram-negative and certain gram-positive bacteria to regulate gene expression. In all forms of bacteria that use quorum sensing, the system is dependent on cell density (Schaefer et al. 1996). Gene regulation occurs as the bacteria release autoinducers, which are signaling molecules that increase in number with cell density. Quorum sensing can be used for a wide range of different processes including the formation of a symbiosis, virulence, the production of antibiotics, the formation of biofilms, motility, and sporulation, amongst others. Although many components of a quorum sensing pathway are unique, they all share the common aspect that they allow for communication that regulates gene expression, and further allows the behavior of all of the cells in a community to be impacted (Miller et al. 2001). Interestingly, the communication is not limited between bacterial cells, but can also impact the cells of a host organism (Visick et al. 2000).
The quorum sensing system in the relationship between Aliivibrio fischeri and the Hawaiian Bobtail squid is the best studied in the world, and is considered the model for understanding how a quorum sensing pathway works. The pathway regulates the genes that cause luminescence. In order for the systems to be successful the high density of bacterial cells in the light organs of the squid are essential (Schaefer et al. 1996). Overall, it is the lux operon that controls quorum sensing in these bacteria, which is made up of a number of different genes. Within this operon there are two units: luxCDABEG and luxR (Lupp et al. 2003). The two main genes involved in any quorum sensing pathway are the luxI and luxR genes. While the luxI gene is responsible for the synthesis of the autoinducer, called N-acyl homoserine lactone (HSL), the R gene is responsible for coding the transcription factor, which then responds to HSL. Aliivibrio fischeri have the genes termed luxI and luxR, which are responsible for synthesizing 3-oxohexanol HSL that then binds to the luxR product. The high cell density required for this pathway to work is necessary, because a critical concentration of the 3-ocohexanol HSL autoinducer needs to be built up for it to bind to the luxR product (Schaefer et al. 1996).
Once critical population density of the Aliivibrio fischeri population has been reached within the light organs of the squid, bioluminescence can occur. An enzyme called luciferase “uses molecular oxygen from the surrounding environment to oxidize both an aliphatic aldehyde and a reduced flavin mononucleotide” (Visick et al 2000, 4578). One of the products of this reaction is an intermediate that is very unstable, and this molecule is responsible for emitting a photon that is a blue-green color, thus producing bioluminescence (Visick et al. 2000). Although the squid do not have control over this reaction, they can control whether or not they show the light. This is done with a shutter that is located within the light organ (Wei et al. 1989).
Also, the squid can eject the bacteria from the light organ, which is actually done approximately every 24 hours. These expulsions can occur, because the light organ has pores that are open to the ocean water around them. Up to 90-95% of the Aliivibrio fischeri within the organ will be expelled by the host squid. The squid only do this expulsion at the onset of the daylight hours, when they return to hide in the sand after being in the ocean water at night. Since the animals are hidden in the sand, bioluminescence is no longer necessary. After the squid has expelled the bacteria, it then needs to regrow them from the few remaining in the crypt, or from bacteria in the surrounding water in a 12 hour period, so that the light organ will be fully functional by the next night. Much about this process is not fully understood, since the metabolic costs of maintaining the bacteria in the crypts are not known. There is, however, the possibility that the squid expel the Aliivibrio fischeri in order to try and select a more competitive symbiont strain (there are many different strains of the bacteria) (Ruby et al. 1998).
Although the luxI gene is the main protein that synthesizes the autoinducer in the quorum sensing system of the Aliivibrio fischeri, there is also a second, less understood gene termed AinS. In their study, Lupp et al. (2003) investigated some of the differences between both the luxI and AinS components of the quorum system. In order to do this, the researchers used both a strain with an AinS mutation, and a strain with an AinS-luxI mutation. The researchers found that while the luxI gene is what primarily drives the production of bioluminescence in the squid, the AinS protein was more important when the bacteria were being grown in culture. Lupp et al. (2005) discovered that the Ain gene allows the Aliivibiro fischeri bacteria to produce bioluminescence at lower densities. They found that mutant strains of Aliivibrio fischeri that contained the luxI gene, but not the AinS gene, were actually not able to colonize the host effectively (Lupp et al. 2003).
Section 2
Include some current research in each topic, with at least one figure showing data.
Section 3
Include some current research in each topic, with at least one figure showing data.
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
[1] Boettcher, K.J., Ruby, E.G. (1990) Depressed Light Emission by Symbiotic Vibrio fischeri of the Sepiolid Squid Euprymna scolopes. Journal of Bacteriology, 172(7): 3701-3706.
[2] Centers for Disease Control and Prevention. (2014) Pseudomonas aeruginosa in Healthcare Settings. http://www.cdc.gov/hai/organisms/pseudomonas.html
[3] Jones, B. W., Nishiguchi, M. K. (2004). Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Marine Biology, 144: 1151-1155.
[4] Lupp, Claudia, Ruby, Edward G. (2005) Vibrio fischeri Uses Two Quorum-Sensing Systems for the Regulation of Early and Late Colonization Factors. Journal of Bacteriology, 187(11): 3620-2629.
[5] Lupp, Claudia, Urbanowski, Mark, Greenberg, E. Peter, Ruby, Edward G. (2003) The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for the persistence in the squid host. Molecular Biology, 50(1): 319-331.
[6] Miller, Melissa B., Bassler, Bonnie L. (2001) Quorum Sensing in Bacteria. Annual Review of Microbiology, 55(1): 165-199.
[7] Ruby, Edward G., Lee, Kyu-Ho. (1998) The Vibrio fischeri-Euprymna scolopes Light Organ Association: Current Ecological Paradigms. Applied and Environmental Microbiology, 64(3): 805-812.
[8] Schaefer, Amy L., Hanzelka, Brian L., Eberhard, Anatol, Greenber, E. P. (1996) Quorum Sensing in Vibrio fischeri: Probing Autoinducer-LuxR Interactions with Autoinducer Analogs. American Society of Microbiology, 178(10): 2897-2901.
[9] Suga, Hiroaki, Smith, Kristina M. (2003) Molecular mechanisms of bacterial quorum sensing as a new drug target. Current Opinion in Chemical Biology, 7(5):586-591.
[10] Urbanczyk H., Ast, J.C., Higgins, M.J., Carson, J., Dunlap, P.V. (2007) Reclassification of the Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibiro logei comg. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. International Journal of Systematic and Evolutionary Microbiology, 57(12): 2823-2829.
[11] Visick, Karen L., Foster, Jamie, Doino Judith, McFall-Ngai, Margaret, Ruby, Edward G. (2000) Vibrio fischeri lux Genes Play an Important Role in Colonization and Development of the Host Light Organ. Journal of Bacteriology, 182(16): 4578-4586.
[12] Visick, Karen L., Ruby, Edward G. (2006) Vibrio fischeri and its host: it takes two to tango. Current Opinion in Microbiology, 9(6):632-638.
[13] Wei, S.L., Young, R. E. (1989) Development of symbiotic bacterial bioluminescence in a nearshore cephalopod, Euprymna scolopes. Marine Biology, 103: 541-546.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
