Anaplasma marginale: Difference between revisions
(edited KMG 7/6/07) |
|||
| Line 51: | Line 51: | ||
|} | |} | ||
Members of the order, Rickettsiales, are small, obligate intracellular bacteria that typically have small genomes due to "reductive evolution" from intracellular parasitism. Because many obligate bacteria are difficult to culture, their need of being grown in a host cell makes it difficult to obtain large amounts of organism-specific DNA necessary for whole genome sequencing. [2] The first complete genome sequencing was done on A. Marginale St. Maries strain. | Members of the order, Rickettsiales, are small, obligate intracellular bacteria that typically have small genomes due to "reductive evolution" from intracellular parasitism. Because many obligate bacteria are difficult to culture, their need of being grown in a host cell makes it difficult to obtain large amounts of organism-specific DNA necessary for whole genome sequencing. [2] The first complete genome sequencing was done on ''A. Marginale St. Maries strain. | ||
*Genes: 1005 | *Genes: 1005 | ||
| Line 64: | Line 64: | ||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
Anaplasma marginale is a pathogenic gram-negative stain bacteria with an outer membrane composed of lipopolysaccharides. Interestingly, several genes for lipopolysaccharide and lipid A biosyntheses were missing. A complete pathway for peptidoglycan synthesis was also not present. “The lack of a traditional cell wall seems to be a common feature for the family Anaplasmataceae, but not from the order Rickettsiales because the family Rickettsiaceae are capable of synthesizing lipopolysaccharide and peptidoglycan. Unlike other members of the family, A. marginale does not seem to be particularly fragile, and may be able to strengthen its cell in an alternative way.” [2] | ''Anaplasma marginale'' is a pathogenic gram-negative stain bacteria with an outer membrane composed of lipopolysaccharides. Interestingly, several genes for lipopolysaccharide and lipid A biosyntheses were missing. A complete pathway for peptidoglycan synthesis was also not present. “The lack of a traditional cell wall seems to be a common feature for the family Anaplasmataceae, but not from the order Rickettsiales because the family Rickettsiaceae are capable of synthesizing lipopolysaccharide and peptidoglycan. Unlike other members of the family, ''A. marginale'' does not seem to be particularly fragile, and may be able to strengthen its cell in an alternative way.” [2] | ||
For energy, Anaplasma marginale undergoes aerobic respiration using the TCA cycle. Anaerobic metabolism, glycolysis, occurs in its cytosol. In vitro tests on organism substrates have shown the use of pyruvate, a glycolytic product, for its metabolism. [3] Even though most of the glycolytic enzymes were detected, a sugar transport system was not found; therefore, the organism may depend more heavily on | For energy, ''Anaplasma marginale'' undergoes aerobic respiration using the TCA cycle. Anaerobic metabolism, glycolysis, occurs in its cytosol. In vitro tests on organism substrates have shown the use of pyruvate, a glycolytic product, for its metabolism. [3] Even though most of the glycolytic enzymes were detected, a sugar transport system was not found; therefore, the organism may depend more heavily on gluconeogenesis for its glucose supply. [2] | ||
Members of its class, Alphaproteobacteria and particularly from the order Rickettsiales, are thought to be the precursors of eukaryotic cell mitochondria. According to the endosymbiotic theory, the mitochondria organelle now existent in eukaryotic cells originated as separate prokaryotic organisms, which were taken inside the cell as endosymbionts. [4] | Members of its class, Alphaproteobacteria and particularly from the order Rickettsiales, are thought to be the precursors of eukaryotic cell mitochondria. According to the endosymbiotic theory, the mitochondria organelle now existent in eukaryotic cells originated as separate prokaryotic organisms, which were taken inside the cell as endosymbionts. [4] | ||
==Ecology== | ==Ecology== | ||
Anaplasma marginale is an intraerythrocytic organism endemic to topical and subtropical regions of the world. Its greatest ecological impact prevails as a worldwide livestock pathogen that can put a constraint | ''Anaplasma marginale'' is an intraerythrocytic organism endemic to topical and subtropical regions of the world. Its greatest ecological impact prevails as a worldwide livestock pathogen that can put a constraint on animal health. In these areas, specific species of cattle ticks are carriers of the bacterium. The organism multiplies in the tick and will pass to later stages of the tick life cycle, but the infection is not passed onto the eggs. As carriers, ticks are unaffected by the bacteria; they carry this infection and infect cattle as a parasite feeding off the blood of the host. Because the adult male tick is more mobile and lives longer than other stages, it is the most likely stage to transmit the disease. Biting flies can transmit the disease but are less efficient vectors than ticks. [7] | ||
Surviving animals are immune to future infections. Calves from immune mothers receive maternal antibodies against A. marginale from | Surviving animals are immune to future infections. Calves from immune mothers receive maternal antibodies against ''A. marginale'' from milk. These calves eventually also develop a lasting immunity; therefore, it is possible to have both ''Anaplasma marginale'' and cattle ticks present in a livestock environment without fear of serious infection outbreaks. [7] | ||
==Pathology== | ==Pathology== | ||
A. marginale is the most pathogenic of its species causing anaplasmosis, which is a form of tick fever carried by specific cattle tick species. The obligate bacterium is an intracellular pathogen. Any stage of the cattle tick’s life cycle can become infected after feeding on an animal carrying the bacteria in its blood. The disease begins its course by invading and multiplying within red blood cells of the host (usually cattle). The gram-negative bacteria produce endotoxins through its lipopolysaccharide outer membrane. As the disease progresses, infected and even uninfected red blood cells are destroyed predominantly in the liver and spleen, resulting in increasing anemia and, in severe cases, death of the host. [7] | ''A. marginale'' is the most pathogenic of its species causing anaplasmosis, which is a form of tick fever carried by specific cattle tick species. The obligate bacterium is an intracellular pathogen. Any stage of the cattle tick’s life cycle can become infected after feeding on an animal carrying the bacteria in its blood. The disease begins its course by invading and multiplying within red blood cells of the host (usually cattle). The gram-negative bacteria produce endotoxins through its lipopolysaccharide outer membrane. As the disease progresses, infected and even uninfected red blood cells are destroyed predominantly in the liver and spleen, resulting in increasing anemia and, in severe cases, death of the host. [7] | ||
Clinical symptoms of infection: | Clinical symptoms of infection: | ||
| Line 87: | Line 87: | ||
* Brown urine due to bile pigments | * Brown urine due to bile pigments | ||
Cattle that recover from anaplasmosis remain carriers of the organism | Cattle that recover from anaplasmosis remain carriers of the organism but are immune to further disease. | ||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
A. marginale can be found worldwide and is the most common tick-borne pathogen of cattle. Studies conducted in this organism are in hopes of finding vaccines against the bacteria and its relatives in the order Rickettsiales, which are pathogens for both animals and humans. | ''A. marginale'' can be found worldwide and is the most common tick-borne pathogen of cattle. Studies conducted in this organism are in hopes of finding vaccines against the bacteria and its relatives in the order Rickettsiales, which are pathogens for both animals and humans. | ||
==Current Research== | ==Current Research== | ||
Because of its damaging, pathogenic nature, much of the current research on this organism is on finding effective vaccines against Anaplasma marginale and its relatives. | Because of its damaging, pathogenic nature, much of the current research on this organism is on finding effective vaccines against ''Anaplasma marginale'' and its relatives. | ||
For efficient tick-borne transmission of the bacteria, there are at least two specific barriers, the midgut and salivary glands. “An inability to colonize the midgut epithelium prevents subsequent development within the salivary glands and thus prevents transmission of the A. Marginale to the host.” [8] Understanding the complexity of the pathogen-tick interaction can open up novel methods of preventing infections. | For efficient tick-borne transmission of the bacteria, there are at least two specific barriers, the midgut and salivary glands. “An inability to colonize the midgut epithelium prevents subsequent development within the salivary glands and thus prevents transmission of the ''A. Marginale'' to the host.” [8] Understanding the complexity of the pathogen-tick interaction can open up novel methods of preventing infections. | ||
“Within the mammalian host, the bacteria generate antigenic variants by changing a surface coat composed of numerous proteins… the surface coat is dominated by two families containing immunodominant proteins: the msp2 superfamily and the msp1 superfamily.” [2] Membrane surface proteins can be useful as microbial identifiers and therefore act as | “Within the mammalian host, the bacteria generate antigenic variants by changing a surface coat composed of numerous proteins… the surface coat is dominated by two families containing immunodominant proteins: the msp2 superfamily and the msp1 superfamily.” [2] Membrane surface proteins can be useful as microbial identifiers and therefore act as antigens eliciting an immune response. Safe vaccine possibilities currently include testing if recombinant major surface protein antigens will produce an effective immune response to protect the animal from future infections. [9] | ||
To add to the difficulty of creating an effective vaccine, the USDA Agriculture Research Service is currently studying the outer membrane protein complex of Anaplasma marginale that includes protection-inducing proteins. The recombinant-based vaccine would be more effective at targeting this cattle disease as it takes into account possible protection mechanisms of the organism. [10] | To add to the difficulty of creating an effective vaccine, the USDA Agriculture Research Service is currently studying the outer membrane protein complex of ''Anaplasma marginale'' that includes protection-inducing proteins. The recombinant-based vaccine would be more effective at targeting this cattle disease as it takes into account possible protection mechanisms of the organism. [10] | ||
==References== | ==References== | ||
Revision as of 14:11, 6 July 2007
A Microbial Biorealm page on the genus Anaplasma marginale
Classification
Higher order taxa
Bacteria; Proteobacteria; Alphaproteobacteria; Rickettsiales; Anaplasmataceae
Species
|
NCBI: Taxonomy |
Anaplasma marginale
Strains:
Anaplasma marginale str. Florida; Anaplasma marginale str. Illinois; Anaplasma marginale str. St. Maries; Anaplasma marginale str. Virginia
Other names:
Anaplasma theileri; Anaplasma rossicum; Anaplasma argentium; Anaplasma theileri Neitz 1957; Anaplasma rossicum Yakimoff and Belawine 1927; Anaplasma argentium Lignieres 1914; Anaplasma marginale Theiler 1910
Description and significance
Anaplasma marginale is the most prevalent tick-borne, livestock pathogen worldwide and is a constraint to animal health. The disease results in significant morbidity and mortality in U.S. cattle and, economically, its exportation. Despite a global impact on animal health, there is still no widely accepted vaccine. Live vaccinations are blood-based and are widely used in tropical countries, but they cannot be licensed in the U.S. because of the risk of transmitting known and unknown pathogens. [1] An effective vaccine for anaplasmosis is a priority for the USDA National Cattlemen’s Beef Association and many other research groups.
A. marginale is the type species for the genus Anaplasma which contains both animal and human pathogens. It is a Rickettsiales obligate intracellular bacterium. These organisms can be easily cultured from the red blood cells of cattle. While erythrocytes seem to be the only site of infection, the bacteria undergo a complex developmental cycle in ticks, and are then transmitted during feeding on host cattle. The genomic information would have a broad applicability to closely related species and organisms within the same order, since members of Rickettsiales are responsible for both animal and human diseases and deaths.
Genome structure
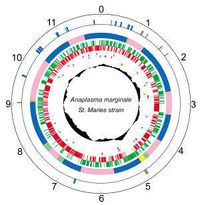
|
Genome: Genome |
Members of the order, Rickettsiales, are small, obligate intracellular bacteria that typically have small genomes due to "reductive evolution" from intracellular parasitism. Because many obligate bacteria are difficult to culture, their need of being grown in a host cell makes it difficult to obtain large amounts of organism-specific DNA necessary for whole genome sequencing. [2] The first complete genome sequencing was done on A. Marginale St. Maries strain.
- Genes: 1005
- Protein coding: 949
- Length: 1,197,687 nt
- Structural RNAs: 40
- GC Content: 49%
- Pseudo genes: 16
- % Coding: 85%
- Topology: circular
[5,6]
Cell structure and metabolism
Anaplasma marginale is a pathogenic gram-negative stain bacteria with an outer membrane composed of lipopolysaccharides. Interestingly, several genes for lipopolysaccharide and lipid A biosyntheses were missing. A complete pathway for peptidoglycan synthesis was also not present. “The lack of a traditional cell wall seems to be a common feature for the family Anaplasmataceae, but not from the order Rickettsiales because the family Rickettsiaceae are capable of synthesizing lipopolysaccharide and peptidoglycan. Unlike other members of the family, A. marginale does not seem to be particularly fragile, and may be able to strengthen its cell in an alternative way.” [2]
For energy, Anaplasma marginale undergoes aerobic respiration using the TCA cycle. Anaerobic metabolism, glycolysis, occurs in its cytosol. In vitro tests on organism substrates have shown the use of pyruvate, a glycolytic product, for its metabolism. [3] Even though most of the glycolytic enzymes were detected, a sugar transport system was not found; therefore, the organism may depend more heavily on gluconeogenesis for its glucose supply. [2]
Members of its class, Alphaproteobacteria and particularly from the order Rickettsiales, are thought to be the precursors of eukaryotic cell mitochondria. According to the endosymbiotic theory, the mitochondria organelle now existent in eukaryotic cells originated as separate prokaryotic organisms, which were taken inside the cell as endosymbionts. [4]
Ecology
Anaplasma marginale is an intraerythrocytic organism endemic to topical and subtropical regions of the world. Its greatest ecological impact prevails as a worldwide livestock pathogen that can put a constraint on animal health. In these areas, specific species of cattle ticks are carriers of the bacterium. The organism multiplies in the tick and will pass to later stages of the tick life cycle, but the infection is not passed onto the eggs. As carriers, ticks are unaffected by the bacteria; they carry this infection and infect cattle as a parasite feeding off the blood of the host. Because the adult male tick is more mobile and lives longer than other stages, it is the most likely stage to transmit the disease. Biting flies can transmit the disease but are less efficient vectors than ticks. [7]
Surviving animals are immune to future infections. Calves from immune mothers receive maternal antibodies against A. marginale from milk. These calves eventually also develop a lasting immunity; therefore, it is possible to have both Anaplasma marginale and cattle ticks present in a livestock environment without fear of serious infection outbreaks. [7]
Pathology
A. marginale is the most pathogenic of its species causing anaplasmosis, which is a form of tick fever carried by specific cattle tick species. The obligate bacterium is an intracellular pathogen. Any stage of the cattle tick’s life cycle can become infected after feeding on an animal carrying the bacteria in its blood. The disease begins its course by invading and multiplying within red blood cells of the host (usually cattle). The gram-negative bacteria produce endotoxins through its lipopolysaccharide outer membrane. As the disease progresses, infected and even uninfected red blood cells are destroyed predominantly in the liver and spleen, resulting in increasing anemia and, in severe cases, death of the host. [7]
Clinical symptoms of infection:
- Transient fever
- Weakness
- Respiratory distress particularly after physical exertions
- Depression
- Loss of appetite
- Jaundice
- Brown urine due to bile pigments
Cattle that recover from anaplasmosis remain carriers of the organism but are immune to further disease.
Application to Biotechnology
A. marginale can be found worldwide and is the most common tick-borne pathogen of cattle. Studies conducted in this organism are in hopes of finding vaccines against the bacteria and its relatives in the order Rickettsiales, which are pathogens for both animals and humans.
Current Research
Because of its damaging, pathogenic nature, much of the current research on this organism is on finding effective vaccines against Anaplasma marginale and its relatives.
For efficient tick-borne transmission of the bacteria, there are at least two specific barriers, the midgut and salivary glands. “An inability to colonize the midgut epithelium prevents subsequent development within the salivary glands and thus prevents transmission of the A. Marginale to the host.” [8] Understanding the complexity of the pathogen-tick interaction can open up novel methods of preventing infections.
“Within the mammalian host, the bacteria generate antigenic variants by changing a surface coat composed of numerous proteins… the surface coat is dominated by two families containing immunodominant proteins: the msp2 superfamily and the msp1 superfamily.” [2] Membrane surface proteins can be useful as microbial identifiers and therefore act as antigens eliciting an immune response. Safe vaccine possibilities currently include testing if recombinant major surface protein antigens will produce an effective immune response to protect the animal from future infections. [9]
To add to the difficulty of creating an effective vaccine, the USDA Agriculture Research Service is currently studying the outer membrane protein complex of Anaplasma marginale that includes protection-inducing proteins. The recombinant-based vaccine would be more effective at targeting this cattle disease as it takes into account possible protection mechanisms of the organism. [10]
References
1) Kocan K.M., de la Fuente J., Guglielmone A.A., Melendez RD. “Antigens and alternatives for control of Anaplasma marginale infection in cattle”. Clinical Microbiology Reviews. 2003 Oct. 16, Vol 4. p. 698-712.
2) Brayton KA, Kappmeyer LS, Herndon DR, Dark MJ, Tibbals DL, Palmer GH, McGuire TC, Knowles DP Jr., "Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins." Proc Natl Acad Sci U S A. 2005 Nov. Vol 102. p 844-9.
3) McHolland, L. E., Caldwell, D. R., "Pyruvate metabolism by Anaplasma marginale in cell-free culture". Canadian Journal of Microbiology. 1999. Vol 45. p 185-189.
4) Margulis, Lynn, “Serial endosymbiotic theory (SET) and composite individuality – Transition from bacterial to eukaryotic genomes.” Microbiology Today. 2004 Nov. Vol 31. p 172-174.
5) NCBI Taxonomy Browser, "Anaplasma marginale" Retrieved 30 April, 2007
6) TGR-CMR, "Anaplasma marginale St. Maries Genome" Retrieved 1 May, 2007
7) HealthGene - Molecular Diagnostic and Research Center. "D425 Anaplasma Marginale"
8) Ueti MW, Reagan JO Jr, Knowles DP Jr, Scoles GA, Shkap V, Palmer GH., "Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale." Infect Immun. 2007 April 9.
9) Kawasaki PM, Kano FS, Tamekuni K, Garcia JL, Marana ER, Vidotto O, Vidotto MC. “Immune response of BALB/c mouse immunized with recombinant MSPs proteins of Anaplasma marginale binding to immunostimulant complex (ISCOM)”. Res Vet Sci. 2007 Mar. 27.
10) USDA, “ARS Project: Outer Membrane Protein Complex of Anaplasma Marginale, Use in Vaccine Development Through Genomics” Start Date: Jul 31, 2003 End Date: May 31, 2008
Edited by Patricia Shih; student of Rachel Larsen and Kit Pogliano
