Antarctica: Difference between revisions
| (269 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
'''<big>Antarctica</big>''' | '''<big>Antarctica</big>''' | ||
| Line 13: | Line 15: | ||
==Slush== | ==Slush== | ||
[[Image: bobslush.jpg|frame|Slush - provided by Dr. Caron of USC | [[Image: bobslush.jpg|left|frame|Slush - provided by Dr. Caron of USC | ||
]] | ]] | ||
| Line 20: | Line 22: | ||
====Location==== | ====Location==== | ||
Slush is located on the southernmost continent on the globe, Antarctica, which covers the South Pole and in the surrounding hydro-areas like the Ross Sea. Each type of ice reflects the age and different forms as well as thickness of ice at different stages of development. Slush refers to recently formed ice and characterized by snow, which is saturated and mixed with water on land or ice surfaces that grow to no more than 10cm thick. | Slush is located on the southernmost continent on the globe, Antarctica, which covers the South Pole and in the surrounding hydro-areas like the Ross Sea. Each type of ice reflects the age and different forms as well as thickness of ice at different stages of development. Slush refers to recently formed ice and is characterized by snow, which is saturated and mixed with water on land or ice surfaces that grow to no more than 10cm thick. [[#References |[1.1]]] | ||
==== Physical Conditions ==== | ==== Physical Conditions ==== | ||
Slush conditions are constantly changing depending on the salinity of the water and the outside temperature. In freshwater, sea slush is more solid and clear while in more saline condition it is more granular and porous. Slush builds up after a heavy rain or snowfall however other climate conditions can affect the slush in several ways, ultimately changing the slush into different categories of ice. For example, sea slush that is allowed to accumulate and freeze into circular pieces of ice would now be categorized as pancake ice. | Slush conditions are constantly changing depending on the salinity of the water and the outside temperature. In freshwater, sea slush is more solid and clear while in more saline condition it is more granular and porous. Slush builds up after a heavy rain or snowfall however other climate conditions can affect the slush in several ways, ultimately changing the slush into different categories of ice. For example, sea slush that is allowed to accumulate and freeze into circular pieces of ice would now be categorized as pancake ice. [[#References |[1.2]]] | ||
==== Affect of Adjacent Communities ==== | ==== Affect of Adjacent Communities ==== | ||
Although Antarctica may seem like a harsh environment, many flora and fauna live on the hostile continent mostly around the costal areas. Antarctica is home many different types of flora, which include two species of flowering plants, three hundred and fifty species of mosses and hundreds of species of algae. Also, several species of fauna live here, mostly inhabiting the neighboring marine environment such as emperor penguins and krill. | Although Antarctica may seem like a harsh environment, many flora and fauna live on the hostile continent mostly around the costal areas. Antarctica is home many different types of flora, which include two species of flowering plants, three hundred and fifty species of mosses and hundreds of species of algae. Also, several species of fauna live here, mostly inhabiting the neighboring marine environment such as emperor penguins and krill. [[#References |[1.3]]] | ||
==== Changing Conditions==== | ==== Changing Conditions==== | ||
Due to the increasing climate change due to human effects such as global warming, Antarctica is sometimes used as a “global warming barometer.” Because of the increasing temperatures, several types of mosses and lichens are able to grow further and further south as increasing periods of thaw occur. | Due to the increasing climate change due to human effects such as global warming, Antarctica is sometimes used as a “global warming barometer.” Because of the increasing temperatures, several types of mosses and lichens are able to grow further and further south as increasing periods of thaw occur. [[#References |[1.3]]] | ||
===Inhabitants=== | ===Inhabitants=== | ||
====Microbes==== | ====Microbes==== | ||
Colwellia polaris, discovered by the LExEn (Life in Extreme Environment) group are gram negative, psychrotolerant, aerobic curved rods, 0.6-0.9 x 0.9-4 | Colwellia polaris, discovered by the LExEn (Life in Extreme Environment) group are gram negative, psychrotolerant, aerobic curved rods, 0.6-0.9 x 0.9-4 micrometers. Cell growth occurs from 4-26°C and pH of 5.0-10.00 while optimum growth occurs around 20°C. [[#References |[1.4]]]. These microorganisms are able to survive and grow at these extreme temperatures because they have thermally adapted to their environment by adopting a variety of strategies such as cold shock proteins as well as structural modifications that allow membrane fluidity to continue in such cold temperatures. In other words, Colwellia polaris are able to deal with the exponential decrease in chemical reaction rates and the increase in membrane rigidity because their proteins and metabolic enzymes are able to function in such cold environments. See interaction with environment and metabolism. | ||
====Other Organisms ==== | ====Other Organisms ==== | ||
| Line 45: | Line 47: | ||
====Environmental Interaction==== | ====Environmental Interaction==== | ||
Colwellia polaris must be able to stop ice from forming around itself which would render it unable to function in its icy slush environment. In order to combat ice buildup, these organisms produce an extracellular substance called an ice binding protein (IBP) which has a high affinity for ice thus inhibiting it from collecting on the organism itself. Ice inoculated with Colwellia polaris | Colwellia polaris must be able to stop ice from forming around itself which would render it unable to function in its icy slush environment. In order to combat ice buildup, these organisms produce an extracellular substance called an ice binding protein (IBP) which has a high affinity for ice thus inhibiting it from collecting on the organism itself. Ice inoculated with Colwellia polaris shows irregular ice growth and pitting on the edges of the ice while uninoculated ice shows no signs of these irregularities. Another related defense instrument is their ice recrystalization inhibition (RI) activity which prevents the formation of larger ice crystals. Ice culture mediums of Colwellia polaris and bovine serum albumin (BSA) were flash frozen and allowed to recrystalize. Due to the RI activity the medium exposed to Colwellia polaris showed smaller, less dense ice crystals. These two extracellular mechanisms allow Colwellia polaris to maintain a fluid environment thus prevent freezing injury to the cell membrane. [[#References |[1.5]]] | ||
====Metabolic Adaptation==== | ====Metabolic Adaptation==== | ||
One specific example is the thermal adaptation of the 71-kDa protein M1 aminopeptidase which is renamed cold-active aminopeptidase (ColAP) for the cold adapted enzyme. This protein has an optimum pH level of 6-8.5 and temperature of | One specific example is the thermal adaptation of the 71-kDa protein M1 aminopeptidase which is renamed cold-active aminopeptidase (ColAP) for the cold adapted enzyme. This protein has an optimum pH level of 6-8.5 and temperature of 19°C. ColAP’s main function is to assist in proteolytic cleavage, which is important in bacterial metabolism. Proteolytic activity aids these organisms in acquiring dissolved organic nutrients such as nitrogen-rich organic compounds. [[#References |[1.6]]] In general, ColAP is able to maintain “normal” function at cold temperatures due to fewer proline residues, fewer ion pairs and a lower hydrophobic residue content, which contribute to its flexibility, which as mentioned before is essential for survival in cold icy environments. The low number of proline residues affect the backbone structure of the protein, which lead to an increase in local motility of the chain. The fewer number of ion pairs increase flexibility by decreasing the number of stabilizing bonds in the protein and lastly, the lower hydrophobic residues indicate an increase in charged residues which increase the hydrosolubilty of the organism and allow a greater accessibility at the surface of the protein. Furthermore, due to these adaptive abnormalities, ColAP is very sensitive to heat thus exhibiting a decreasing half-life with increasing temperature. For example, at 0°C, the enzyme half-life was measured to be between 18 and 45 hours while at 50°C the half-life was measured to be only 5 minutes. [[#References |[1.7]]] | ||
[[# | [[#Slush |Back to top]] | ||
==Lake Vostok== | ==Lake Vostok== | ||
[[Image: new_vostok_cartoon_high.jpg|left|frame|Used with kind permission from [http://www.ldeo.columbia.edu/~mstuding/vostok.html Michael Studinger] | [[Image: new_vostok_cartoon_high.jpg|left|frame|Used with kind permission from [http://www.ldeo.columbia.edu/~mstuding/vostok.html Dr Michael Studinger] | ||
]] | ]] | ||
===Description of Niche=== | ===Description of Niche=== | ||
Lake Vostok lies beneath the Russian Research Station Vostok for which it is named. This subglacial lake has been studied since the late 1960’s yet no one has actually sampled the water within it directly. It is the Largest of the approximately 150+ subglacial lakes | Lake Vostok lies beneath the Russian Research Station Vostok for which it is named. This subglacial lake has been studied since the late 1960’s yet no one has actually sampled the water within it directly. It is the Largest of the approximately 150+ subglacial lakes [[#References |[2.1]]] comparable in size to Lake Ontario with approximately 5000 km<sup>3</sup> of water.[[#References |[2.2, 2.3]]] It is thought that Lake Vostok has been continuously isolated from the earth’s atmosphere for the past 15-30 million years, also rendering it cut off from new carbon sources as well as light[[#References |[2.3]]] making it a very unique environment. The glacial ice moves across the lake at a speed of ~3m per year in an easterly direction.[[#References |[2.2]]] | ||
====Location==== | ====Location==== | ||
[[Image: Vostok_survey_are.gif |frame |Used with kind permission from [http://www.ldeo.columbia.edu/~mstuding/vostok.html Dr Michael Studinger]] | |||
Lake Vostok is located in East Antarctica under approximately 4km of ice. The ice sheath varies in thickness from the northern to the southern region. | Lake Vostok is located in East Antarctica under approximately 4km of ice. The ice sheath varies in thickness from the northern to the southern region.[[#References |[2.2]]] | ||
====Physical Conditions==== | ====Physical Conditions==== | ||
Lake Vostok, like many other subglacial lakes holds many extreme physical conditions, ranging from high pressure ~350 atm, low temperatures (between - | Lake Vostok, like many other subglacial lakes holds many extreme physical conditions, ranging from high pressure ~350 atm, low temperatures (between -3°C and 8°C), and permanent darkness. [[#References |[2.4]]] The high pressure exuded from the ice above Lake Vostok keeps is what keeps the lake water in a liquid state, since water freezes at a lower temperature when under high pressure.[[#References |[2.5]]] | ||
====Affect of Adjacent Communities==== | ====Affect of Adjacent Communities==== | ||
Lake Vostok was thought to have been not only isolated from the 20th century atmosphere but also from any other surrounding environments. This thinking has now changed as recent imaging studies have shown that these subglacial lakes are potentially connected through a network of rivers. This makes sterile drilling all the more important, since contamination of one subglacial lake increases the chances of other adjacent subglacial lakes becoming contaminated.[[#References |[2.2]]] Also, the actual lake itself is in constant balance with the accretion ice above it. The ice melts in the northern part of Lake Vostok possibly delivering nutrients and other microorganisms into the lake.[[#References |[2.2]]] | |||
As the ice in the northern region of Lake Vostok melts it sinks to the lake floor, where it is warmed via geothermal activity and/or pressure. As the water temperature increases, causing the lake water to become less dense it rise to the surface in the south. Once the water has resurfaced in the south it freezes and becomes part of the accreted ice. This water movement is important in considering the ice core samples. As the water rises to the surface it can bring some of the sediment and the organisms which reside on the lake floor or event throughout the varying depths with it causing it to become trapped in the ice. The coring at Lake Vostok has penetrated into the accretion zone in the southern portion of the lake. (The accretion zone is the lowest 210m, and the core sample was taken from 150m above the lake)[[#References |[2.4]]] | |||
As the ice moves across any sediment a small amount of the it becomes entrapped.[[#References |[2.6]]] According to Christner et al., this provides a mechanism by which organism can get transferred into Lake Vostok. | |||
[[Image: Vostok.jpg|frame|Vostok Station | [[Image: Vostok.jpg|frame| left|Vostok Station | ||
Image from National Science Foundation]] | Image from National Science Foundation]] | ||
====Changing Conditions==== | ====Changing Conditions==== | ||
Dependent on the depth of the water the physical conditions of Lake Vostok can | Dependent on the depth of the water the physical conditions of Lake Vostok can vary. Lake Vostok has been previously thought to be an area without any seismic activity – this has now changed. A low amount of seismic activity has been noted. The seismic activity can release organisms into the lake as well as provide a source of heat and nutrition. Furthermore, microseismic activity has been recorded nearby the Vostok station which cold drive convection movement within the Lake.[[#References |[2.2]]] | ||
===Inhabitants=== | ===Inhabitants=== | ||
Currently there is no concrete knowledge on the type of organisms which reside in Lake Vostok. There are many stipulations on the types of organisms, if any, that might be found within any of the subglacial lakes including Lake Vostok. The organisms speculated to inhabit this niche are most likely extremophiles, tolerant of extremely low temperatures (<0°C). It is further postulated that these organisms would probably have an adaptation to high pressure. In recent years continued coring has indeed brought evidence of such microbes. According to Karl, et al. the organisms found within one of the core samples were found to be oligotrophic. Oligotrophic organisms live with minimal nutrients, low biomass, and also a low energy flux,[[#References |[2.5]]] which would be consistent with the conditions wihting the lake. | |||
In one experiment core ice from section 3593 was melted and samples of the melt ice were spread on agar plates, enriched with various nutrients and incubated at 25°C. Four colonies were grown and 16S rDNA comparison was performed. This analysis showed that the microbes, which had been cultured, have similar 16S sequences. Their nearest phylogenetic neighbors are ''Brachybacterium conglomeratum'' (found in cheese), ''[[Sphingomonas]]'' ''sp.'' (found in Guliya ice core), ''Paenibacillus amylolyticus'' (found in soil), ''[[Methylobacterium]] sp.'' (found as biofilm on cooling fan), and one unidentified organism.[[#References |[2.6]]] | |||
Another study found fourteen different isolates which had been cultured on agar enriched with different nutrients. Among these fourteen separate colonies ''Cruptococcus sp.'' and ''Rhodoturula sp.''(both in the yeast family) were identified as well as ''Subtercola sp.'', ''sphingomaonas sp.'' leaving nine remaining to be identified.[[#References |[2.7]]] | |||
====Microbes==== | ====Microbes==== | ||
Evidence from past research suggests the presence of thermophilic chemoautotrophic microorganisms in Lake Vostok. [[#References |[2.3]]] As of yet we can still only stipulate what types of organisms can be found within the actual Lake as all of the coring samples are from accretion ice. Also, there is a large chance for organisms found within the ice cores to be contaminant, even after the large amount of precautions taken to minimize the risk thereof. So far Researchers have isolated a few novel species which might be found within Lake Vostok: | |||
Evidence suggests the presence of thermophilic chemoautotrophic microorganisms in Lake Vostok. | |||
* ''Hydrogenophilus gen. nov.'' is a rod shaped, non sporulating, Gram-negative bacterium, which is a chemolithoautotrophe. These bacteria utilize H<sup>+</sup> as their electron donor, with CO<sub>2</sub> as their electron acceptor.[[#References |[2.8]]] | |||
''Hydrogenophilus thermoluteolus'' | * ''Hydrogenophilus thermoluteolus sp. nov.'' is a heterotrophic bacterium, which grows best at temperatures between 50-52°C and a pH of 7. When grown in colonies it has a dull yellow color. It uses acetate, propionate butyrate, succinate, DL-lactate, pyruvate and α-ketoglutarate as both electron donors and as their carbon source. It uses Ammonium ions, nitrate ions and urea as its nitrogen sources. [[#References |[2.8]]] | ||
====Environmental Interaction==== | ====Environmental Interaction==== | ||
It has been thought that some of the organisms which reside in such extreme cold have an ability which allows them to change the physical structure of the ice around them. Also, these conditions warrant a slow metabolism as well as an alternate energy source. | It has been thought that some of the organisms which reside in such extreme cold have an ability which allows them to change the physical structure of the ice around them. Also, these conditions warrant a slow metabolism as well as an alternate energy source. | ||
Ice binding proteins, which inhibit ice- | Ice binding proteins, which inhibit ice-recrystallization have been isolated out of core samples at Lake Vostok.[[#References |[2.7]]] Raymond et al. discuss the possibility of bacteria and other microbes forming channels through the ice. These channels would be filled with liquid water as the ice-binding proteins would inhibit the recrystallization. This would be a ground where potential communities of microbes could survive. It is also thought that other microbes, which do not carry the gene for the ice-binding proteins could live along side those that posses this unique ability. The interaction and habitat formation is thought to be similar to that of soil bacteria.[[#References |[2.7]]] | ||
[[#Slush |Back to top]] | [[#Slush |Back to top]] | ||
| Line 122: | Line 126: | ||
====Physical Conditions==== | ====Physical Conditions==== | ||
The average temperature of ice in the Ross Ice Shelf is -2. | The average temperature of ice in the Ross Ice Shelf is -2.13 to -2.16° C, slightly colder than -2°C, the temperature at which seawater freezes. The Ross Ice Shelf receives 202.5mm of rainfall per year on average. The average temperature of the Ross Sea is -1.9°C, and it is a salt water sea [[#References |[3.2]]]. | ||
====Changing Conditions==== | ====Changing Conditions==== | ||
The Ross Dependency region receives the most rain between February and June, an average of 22.5mm/month. Between July and January the amount of rainfall severely decreases to about 12.7mm/month. The average temperature, between - | The Ross Dependency region receives the most rain between February and June, an average of 22.5mm/month. Between July and January the amount of rainfall severely decreases to about 12.7mm/month. The average temperature, between -2°C and -10°C, is highest between November and February. From March to October, the average temperature is between -18°C and -26°C [[#References |[3.5]]]. | ||
The Ross Dependency and all of Antarctica experiences 6 months of continuous sunlight and 6 months of continuous darkness each year. | The Ross Dependency and all of Antarctica experiences 6 months of continuous sunlight and 6 months of continuous darkness each year. | ||
| Line 133: | Line 137: | ||
====Microbes==== | ====Microbes==== | ||
Many types of phytoplankton live in the Ross Dependency area. These include several species of diatoms, haptophytes, dinoflagellates, and cryptophytes | Many types of phytoplankton live in the Ross Dependency area. These include several species of diatoms, haptophytes, dinoflagellates, and cryptophytes [[#References |[3.4]]]. | ||
Some of the diatoms that live there are Corethron coriophyllum, Pseudonitschia, Fragilariopsis, Rhizosolenia, and Thalassiosira. | Some of the diatoms that live there are Corethron coriophyllum, Pseudonitschia, Fragilariopsis, Rhizosolenia, and Thalassiosira. | ||
Diatoms like Fragilariopsis and Pseudoitschia are found in extensive blooms near ice edges in the summer months | Diatoms like Fragilariopsis and Pseudoitschia are found in extensive blooms near ice edges in the summer months [[#References |[3.4]]]. | ||
Cryptophytes can also be found in large blooms in the Ross Sea. | Cryptophytes can also be found in large blooms in the Ross Sea. | ||
One of the most studied haptophyte that lives in the Ross Dependency is Phaeocystis antarctica. P. antarctica generally live in hollow colonies though solitary cells have been found, as well. Living in colonies helps this bacterium evade predators as single cells are more likely to be ingested. This microorganism, as well as many other bacteria that live in the Ross Dependency, is most prevalent in spring and populations decline drastically in the summer months | One of the most studied haptophyte that lives in the Ross Dependency is Phaeocystis antarctica. P. antarctica generally live in hollow colonies though solitary cells have been found, as well. Living in colonies helps this bacterium evade predators as single cells are more likely to be ingested. This microorganism, as well as many other bacteria that live in the Ross Dependency, is most prevalent in spring and populations decline drastically in the summer months [[#References |[3.1]]]. | ||
The tidal zone of the Ross Ice Shelf has a biofilm made of diatoms and cyanobacteria. | The tidal zone of the Ross Ice Shelf has a biofilm made of diatoms and cyanobacteria. | ||
| Line 143: | Line 147: | ||
There are no land based vertebrate animals naturally present in the Ross Dependency, or even in Antarctica. Small invertebrate animals inhabit the island. No trees or bushes can survive there, however around 350 species of mosses, lichens and algae live around Antarctica. | There are no land based vertebrate animals naturally present in the Ross Dependency, or even in Antarctica. Small invertebrate animals inhabit the island. No trees or bushes can survive there, however around 350 species of mosses, lichens and algae live around Antarctica. | ||
In addition to the microbes, many types of marine life including larger plankton, krill, fish, seal, penguins, and whales live in the Ross Sea. Phytoplankton are a direct energy source for some of these organisms. Bacteria is the primary food source for microzooplankton. Ice Krill and plankton eat diatoms, and Antarctic toothfish often consume P. Antarctica | In addition to the microbes, many types of marine life including larger plankton, krill, fish, seal, penguins, and whales live in the Ross Sea. Phytoplankton are a direct energy source for some of these organisms. Bacteria is the primary food source for microzooplankton. Ice Krill and plankton eat diatoms, and Antarctic toothfish often consume P. Antarctica [[#References |[3.2]]]. | ||
====Metabolic Adaptations==== | ====Metabolic Adaptations==== | ||
Scientists have found that Antarctic ice, including the Ross Ice Shelf, contains extensive criss-crossing acidic "veins" that can have pH as low as 0. Microbes who live in these veins are thought to extract their energy from the acid surrounding them | Scientists have found that Antarctic ice, including the Ross Ice Shelf, contains extensive criss-crossing acidic "veins" that can have pH as low as 0. Microbes who live in these veins are thought to extract their energy from the acid surrounding them [[#References |[3.7]]]. These acidophiles must also protect themselves from too much acid. In order to keep their intracellular pH ideal, some microbes maintain a positive surface membrane charge, have a membrane that is impermeable to protons, or synthesize proteins that remove protons from the cell. Some microbes may also form spores if they can't regulate intracellular acidity well enough [[#References |[3.7]]]. Unlike an activly metabolizing organism, spores do not need to regulate intracellular pH very tightly. | ||
The extremely low temperatures of the South Pole make it very challenging for microbial life to thrive there. Because of these harsh conditions, microbes that live there often have very low metabolic and division rates; some even shut down all unnecessary energy consumption when the temperatures drop. A microbe may have other responses to the cold, including: an increase in production of chaperones and other stress proteins, express starvation genes, or synthesis of "antifreeze proteins" to protect their cytoplasm from freezing | The extremely low temperatures of the South Pole make it very challenging for microbial life to thrive there. Because of these harsh conditions, microbes that live there often have very low metabolic and division rates; some even shut down all unnecessary energy consumption when the temperatures drop. A microbe may have other responses to the cold, including: an increase in production of chaperones and other stress proteins, express starvation genes, or synthesis of "antifreeze proteins" to protect their cytoplasm from freezing [[#References |[3.7]]]. | ||
Addtionally, microbes living in the Ross Dependency necessarily have a greater percentage of unsaturated fatty acids in their membranes than do warmer-dwelling microbes in order to retain membrane fluidity | Addtionally, microbes living in the Ross Dependency necessarily have a greater percentage of unsaturated fatty acids in their membranes than do warmer-dwelling microbes in order to retain membrane fluidity [[#References |[3.7]]]. | ||
====Environmental Interactions==== | ====Environmental Interactions==== | ||
P. antarctica produces a lot of dimethylsulfide. This is a volatile substance that is necessary for cloud formation. As dimethylsulfide rises into the atmosphere it transforms into aerosols that attract molecules of water, creating clouds | P. antarctica produces a lot of dimethylsulfide. This is a volatile substance that is necessary for cloud formation. As dimethylsulfide rises into the atmosphere it transforms into aerosols that attract molecules of water, creating clouds [[#References |[3.3]]]. | ||
Phytoplankton in the Ross Dependency produce chlorophyll. The concentration of chlorophyll in a region can be used to estimate the phytoplankton population at the time. | Phytoplankton in the Ross Dependency produce chlorophyll. The concentration of chlorophyll in a region can be used to estimate the phytoplankton population at the time. | ||
Diatoms and haptophytes also synthesize carbon biomass. This organic matter sinks to a depth of 500 meters by the natural sinking of phytoplankton. | Diatoms and haptophytes also synthesize carbon biomass. This organic matter sinks to a depth of 500 meters by the natural sinking of phytoplankton. | ||
[[# | [[#Slush |Back to top]] | ||
==Soil== | ==Soil== | ||
| Line 167: | Line 171: | ||
Most Antarctic soil is covered with ice from meters to miles thick; only 2% of soil is exposed. | Most Antarctic soil is covered with ice from meters to miles thick; only 2% of soil is exposed. [[#References|[4.1]]] Inland deserts constitute most of these bare regions, such as the McMurdo Dry Valleys, which receive little to no precipitation. In the Dry Valleys, the equivalent of only 50 mm of rainfall accumulates every year. Temperatures up to a few degrees above freezing, and as low as -89.2° C have been recorded in the center of the continent. [[#References|[4.2]]] | ||
====Location==== | ====Location==== | ||
Exposed soil is mostly found in the interior of Antarctica in the Mcmurdo Dry Valleys. The coordinates of this region are: 77"00'S, 162O52'E. | Exposed soil is mostly found in the interior of Antarctica in the Mcmurdo Dry Valleys. The coordinates of this region are: 77"00'S, 162O52'E. [[#References|[4.3]]] | ||
Soil is also found in Cryoconites. (See Physical Conditions for more information about Cryoconites.) | Soil is also found in [http://www-es.s.chiba-u.ac.jp/~takeuchi/cryoconite.html Cryoconites]. (See Physical Conditions for more information about Cryoconites.) | ||
====Physical Conditions==== | ====Physical Conditions==== | ||
[[Image:CCH_proc.jpg|left|frame| Diagram of open Cryoconite. Reproduced/modified by permission of American Geophysical Union. [[#References|[4.29]]] ]] | |||
The soil in Antarctica is freezing, arid, low in clay content and organic matter, and high in [http://en.wikipedia.org/wiki/Salinity salinity]. It is around 96% sand. There is very little bio-available water. Due to the lack of precipitation, water does not leach [http://en.wikipedia.org/wiki/Cations cations] from the earth, leaving it with an alkaline pH. [[#References|[4.5]]] Scientists measured a pH level of 8.51 (+ or - 0.07) in places containing living organisms, though, this is lower than the average soil pH. [[#References|[4.22]]] | |||
Although not as common as other soil conditions in Antarctica, geothermal activity brings dirt temperature up to 42-60° C, and pH down to 4.5-7.5. Steam vents that produce the heat also add moisture to the earth. They can be found on northwest slope of Mount Melbourne. | Cryoconites are water and sediment filled hollows on the surfaces or edges of [http://en.wikipedia.org/wiki/Glacier glaciers]. The water and soil mixture periodically freezes and melts. The dark sediment absorbs more light than the white ice and snow, causing it to heat up and melt the surrounding ice. This forms pockets of subsurface liquid water with sediment at the bottom. In the summer, when the covering ice melts, the depressions are open to the atmosphere and receive minerals and microorganisms blown by the wind. The sediment in cryoconites is submerged in water, but is chemically unique from glacial runoff and water found in lakes and rivers. [[#References|[4.4]]] | ||
Although not as common as other soil conditions in Antarctica, [http://en.wikipedia.org/wiki/Geothermal_(geology) geothermal] activity brings dirt temperature up to 42-60° C, and pH down to 4.5-7.5. Steam vents that produce the heat also add moisture to the earth. They can be found on northwest slope of Mount Melbourne. [[#References|[4.6]]] | |||
====Affect of Adjacent Communities==== | ====Affect of Adjacent Communities==== | ||
The Mcmurdo Dry Valleys also hold rivers and lakes, and are bounded by glaciers and snow pack. Microbes in these niches can interact with those in the dirt. | The Mcmurdo Dry Valleys also hold rivers and lakes, and are bounded by glaciers and snow pack. Microbes in these niches can interact with those in the dirt. [[#References|[4.3]]] | ||
Wind plays a key role in the movement and dispersal of organic matter. | Wind plays a key role in the movement and dispersal of organic matter. [[#References|[4.20]]] Dust from the bare land in the McMurdo Valleys is lifted and blown by the wind to rivers, lakes, and glaciers with cryoconites. The dust carries microbes and minerals with it. The microbes caught in cryoconites and in snow can be flushed into lakes by glacial melt. Evaporation can also pick up microorganisms in water and precipitate them elsewhere. These changes in environment promote genetic mixing. [[#References|[4.4]]] | ||
One research group suggested that cyanobacteria originate in bodies of water and adapt as they move from water, to mud, to dry, desert soil. They reason that Beacon Valley has a very low cyanobacteria population because it has no lakes or ponds, while in Miers Valley, cyanobacteria florish because bodies of water are present there. | One research group suggested that cyanobacteria originate in bodies of water and adapt as they move from water, to mud, to dry, desert soil. They reason that Beacon Valley has a very low cyanobacteria population because it has no lakes or ponds, while in Miers Valley, cyanobacteria florish because bodies of water are present there. [[#References|[4.7]]] | ||
====Changing Conditions==== | ====Changing Conditions==== | ||
The Antarctic soil environment changes seasonally. In the McMurdo Dry Valleys during winter, Antarctica is almost continuously dark, the wind can reach speeds of 110 km/h, and the air temperature drops to -60° C. In the summer there is nearly constant daylight, and the temperature oscillates between -35° C and 3° C, depending on cloud cover and wind chill. Most precipitation falls in the summer. When in prolonged direct sunlight, soil and rocks can heat up to 10° C above ambient temperature. | The Antarctic soil environment changes seasonally. In the McMurdo Dry Valleys during winter, Antarctica is almost continuously dark, the wind can reach speeds of 110 km/h, and the air temperature drops to -60° C. In the summer there is nearly constant daylight, and the temperature oscillates between -35° C and 3° C, depending on cloud cover and wind chill. Most precipitation falls in the summer. When in prolonged direct sunlight, soil and rocks can heat up to 10° C above ambient temperature. [[#References|[4.8]]] Lots of sunlight also brings increased [http://en.wikipedia.org/wiki/Solar_radiation solar radiation], which can damage DNA. The problem is exascerbated by the large [http://en.wikipedia.org/wiki/Ozone_depletion hole in the ozone layer] over Antarctica, which admits even more UV rays. [[#References|[4.9]]] | ||
The environment also varies from place to place. In one study looking at the correlation between the living soil communities and abiotic soil composition, soil chemistry and elevation data were collected. The information showed that the amount of nitrogen found in soil samples decreased as elevation increased. This | The environment also varies from place to place. In one study looking at the correlation between the living soil communities and abiotic soil composition, soil chemistry and elevation data were collected. The information showed that the amount of nitrogen found in soil samples decreased as elevation increased. This implies that fewer organisms live at higher altitudes, which are colder and tend to have higher salinity. Overall, populations have been described as "patchy," with highly populated regions right next to areas with no life at all because of the varied sediment nutrient content. [[#References|[4.8]]] | ||
Scientists are now noticing some effects of oil and other hydrocarbon pollution on the | Scientists are now noticing some effects of oil and other [http://en.wikipedia.org/wiki/Hydrocarbon hydrocarbon] pollution on the microbe communities. The waste has proved beneficial for some bacterial, and detrimental for others. The overall diversity decreases when hydrocarbon contamination is present. [[#References|[4.26]]] | ||
===Inhabitants=== | ===Inhabitants=== | ||
| Line 206: | Line 211: | ||
[[Image:Lyngb3.jpg|frame|Filamentous Cyanobacteria without heterocysts. The genus <i>Plectonema</i> belongs in this category. © 1997, Microbial Diversity]] | [[Image:Lyngb3.jpg|frame|Filamentous Cyanobacteria without heterocysts. The genus <i>Plectonema</i> belongs in this category. © 1997, Microbial Diversity]] | ||
Colonies of bacteria have been found growing in the pores of sandstone rock to avoid exposure to the rapidly changing environment. Organisms that live inside rocks this way are called cryptoendoliths. Endolithic communities are thought to comprise the main forms of life in the polar deserts. | Colonies of bacteria have been found growing in the pores of sandstone rock to avoid exposure to the rapidly changing environment. Organisms that live inside rocks this way are called cryptoendoliths. Endolithic communities are thought to comprise the main forms of life in the polar deserts. | ||
In one study, researchers isolated primarily Cyanobacteria, extremophilic bacteria. and microbial lichen, all primary producing, endolithic organisms, in the soil and rocks of the McMurdo Dry Valleys. To catagorize each individual microbe, they examined their small-subunit (SSU) rRNA genes and matched them with the genes of known microorganisms. They discovered three specific bacteria that were most abundant in their samples. The first, comprising 30% of the sample, is most closely related to the <i>Plectonema</i> genus, (97% rRNA sequence identity). The second, 31% of the sample, is similar to <i>Blastomonas ursincola</i>, (94% rRNA sequence identity). It is representative of α-Proteobacteria, a phylum of aerobic, anoxygenic, phototrophs. The third, making up 26% of the sample, is a microbe with no very close relatives, but with a distant relative from the <i>Deinococcus</i> genus, (90% nucleotide sequence identity). It can be categorized in its own clade called Deinococcus-Thermus. | In one study, researchers isolated primarily [http://en.wikipedia.org/wiki/Cyanobacteria Cyanobacteria], extremophilic bacteria. and microbial [http://en.wikipedia.org/wiki/Lichen lichen], all primary producing, endolithic organisms, in the soil and rocks of the McMurdo Dry Valleys. To catagorize each individual microbe, they examined their small-subunit (SSU) [http://en.wikipedia.org/wiki/RRNA rRNA] genes and matched them with the genes of known microorganisms. They discovered three specific bacteria that were most abundant in their samples. The first, comprising 30% of the sample, is most closely related to the <i>Plectonema</i> genus, (97% rRNA sequence identity). The second, 31% of the sample, is similar to <i>Blastomonas ursincola</i>, (94% rRNA sequence identity). It is representative of α-Proteobacteria, a phylum of aerobic, anoxygenic, [http://en.wikipedia.org/wiki/Phototroph phototrophs]. The third, making up 26% of the sample, is a microbe with no very close relatives, but with a distant relative from the <i>[[Deinococcus]]</i> genus, (90% nucleotide sequence identity). It can be categorized in its own clade called Deinococcus-Thermus. [[#References|[4.10]]] Bacteria of the Deinococcus-Thermus phylum are incredibly resistant: they can withstand radiation and extreme temperatures; survive in a vacuum, through droughts and famines; and consume nuclear waste. This phylum is also closely related to Cyanobacteria. [[#References|[4.27]]] | ||
They found the remaining percentage to be representatives of green nonsulfur bacteria, Cytophagales, Acidobacteria, and Actinobacteria (6% of clones). Acidobacteria are a phylum of acidophilic bacteria. | They found the remaining percentage to be representatives of green nonsulfur bacteria, Cytophagales, Acidobacteria, and Actinobacteria (6% of clones). [http://en.wikipedia.org/wiki/Acidobacteria Acidobacteria] are a phylum of acidophilic bacteria. [[#References|[4.10]]] [http://en.wikipedia.org/wiki/Actinobacteria Actinobacteria] are gram-postitive bacteria that can decompose strong molecules such as [http://en.wikipedia.org/wiki/Chitin chitin] and [http://en.wikipedia.org/wiki/Cellulose cellulose]. Most are [http://en.wikipedia.org/wiki/Aerobic_organism aerobic], but a few are [http://en.wikipedia.org/wiki/Anaerobic_organisms anaerobic]. Some Actinobacteria can produce exospores. [[#References|[4.11]]] Cyanobacteria, another phylum also known as blue-green algae, obtain energy through photosynthesis. They are [http://en.wikipedia.org/wiki/Gram-negative Gram-negative], lack flagella, and usually have thick cell walls. [[#References|[4.12]]] | ||
In the same study, the dry soil was also found to contain organisms that have rRNA representative of green algae, fungi, and chloroplasts. The isolated fungus is closely related to ascomycete fungus Texosporium sancti-jacobi, while the algae is very similar to Trebouxia jamesii. Each of these species has been identified as a kind of fungi and algae that participates in lichen symbiosis. Because these specific species made up over 70% of the clones produced through PCR for this experiment, they are thought to make up the dominant lichen in the community. | In the same study, the dry soil was also found to contain organisms that have rRNA representative of green algae, fungi, and chloroplasts. The isolated fungus is closely related to ascomycete fungus <i>Texosporium sancti-jacobi</i>, while the algae is very similar to <i>Trebouxia jamesii</i>. Each of these species has been identified as a kind of fungi and algae that participates in lichen symbiosis. Because these specific species made up over 70% of the clones produced through [http://en.wikipedia.org/wiki/PCR PCR] for this experiment, they are thought to make up the dominant lichen in the community. | ||
Those performing this study mentioned that while they did not uncover all the species in Antarctic soils, they do believe that that have found the main contributors to the energy cycle. | Those performing this study mentioned that while they did not uncover all the species in Antarctic soils, they do believe that that have found the main contributors to the energy cycle. [[#References|[4.10]]] | ||
In the McMurdo Dry Valleys, Cryoconites support cyanobacteria, photosynthetic algae, heterotrophic bacteria, tardigrades, rotifers, and other microorganisms. | In the McMurdo Dry Valleys, Cryoconites support cyanobacteria, photosynthetic algae, heterotrophic bacteria, [http://en.wikipedia.org/wiki/Tardigrade tardigrades], [http://en.wikipedia.org/wiki/Rotifer rotifers], and other microorganisms. [[#References|[4.4]]] | ||
A few novel species of microbes have been isolated and identified around steam vents in Antarctica. The first of these is <i>Brevibacillus levickii</i>. Another is most closely related to <i>Alicyclobacillus pomorum</i> (91% similarity). The genus <i>Alicyclobacillus</i> is a group of Gram-positive, heterotrophic, bacteria that form endospores. Both species are thermophilic, and, thus, capable of living at very high temperatures. | A few novel species of microbes have been isolated and identified around steam vents in Antarctica. The first of these is <i>Brevibacillus levickii</i>. Another is most closely related to <i>Alicyclobacillus pomorum</i> (91% similarity). The genus <i>Alicyclobacillus</i> is a group of [http://en.wikipedia.org/wiki/Gram-positive Gram-positive], heterotrophic, bacteria that form [http://en.wikipedia.org/wiki/Endospore endospores]. Both species are [http://en.wikipedia.org/wiki/Thermophile thermophilic], and, thus, capable of living at very high temperatures. [[#References|[4.6]]] | ||
Populations of Archaea are | Populations of Archaea are small and belong to the Group II low-temperature Crenarchaeotes. [[#References|[4.13]]] | ||
Early research suggested that diversity was extremely low in the desert inland due to severe environmental conditions, but new experimental methods have shown that the microbial biomass in Antarctic soil is between three to four magnitudes higher than previous estimates, and may have considerably higher diversity, though less diversity than that of milder climates. | Early research suggested that diversity was extremely low in the desert inland due to severe environmental conditions, but new experimental methods have shown that the microbial biomass in Antarctic soil is between three to four magnitudes higher than previous estimates, and may have considerably higher diversity, though less diversity than that of milder climates. [[#References|[4.13]]] | ||
Specific rRNA sequences, or related species, are only found in very small sections of earth, and not found anywhere else. This implies a large degree of isolation from one community to the next. It also supports the idea that biomass is found in patches surrounded by dead areas. | Specific rRNA sequences, or related species, are only found in very small sections of earth, and not found anywhere else. This implies a large degree of isolation from one community to the next. It also supports the idea that biomass is found in patches surrounded by dead areas. [[#References|[4.24]]] | ||
====Other Organisms==== | ====Other Organisms==== | ||
Certain species of nematodes, springtails, and mites also make the soil and rocks of the Antarctic their home. Those mosses and arthropods may only be able to survive inland in the summer if soil moisture is high enough. | Certain species of [http://en.wikipedia.org/wiki/Nematode nematodes], [http://en.wikipedia.org/wiki/Springtail springtails], and [http://en.wikipedia.org/wiki/Mites mites] also make the soil and rocks of the Antarctic their home. Those mosses and arthropods may only be able to survive inland in the summer if soil moisture is high enough. [[#References|[4.13]]] Nematodes feed on bacteria, but also provide nutrients for bacteria when they die. [[#References|[4.15]]] Other invertebrates live in the coastal regions, but are not able to survive in the central portion of Antarctica. | ||
====Microbe Interaction==== | ====Microbe Interaction==== | ||
Population density is not high in the desert communities, but since nutrients are so scarce, some competition does occur between different bacterial species. Yet, abiotic factors, such a temperature and soil moisture, are more likely to determine community structure than interaction between organisms (competition, herbivory, predation). | Population density is not high in the desert communities, but since nutrients are so scarce, some competition does occur between different bacterial species. Yet, abiotic factors, such a temperature and soil moisture, are more likely to determine community structure than interaction between organisms (competition, herbivory, predation). [[#References|[4.13]]] In fact, biodiversity and population density appears to be most dependent on the amount of moisture in the earth. [[#References|[4.21]]] | ||
As noted above, some species of lichens inhabit Antarctica. Lichens are a mutually beneficial arrangement of fungi and single-celled algae called a symbiosis. Together they form a larger, multicellular structure. Fungi cling to rocks to form a foundation on which to live, and compete with other fungi for space. The fungi also give protection and supply water and minerals, while the algae provide nutrients made by photosynthesis. | As noted above, some species of lichens inhabit Antarctica. Lichens are a mutually beneficial arrangement of fungi and single-celled algae called a symbiosis. Together they form a larger, multicellular structure. Fungi cling to rocks to form a foundation on which to live, and compete with other fungi for space. The fungi also give protection and supply water and minerals, while the algae provide nutrients made by [http://en.wikipedia.org/wiki/Photosynthesis photosynthesis]. [[#References|[4.14]]] | ||
The heterotrophic bacteria eat other microbes, including cyanobacteria. | The [http://en.wikipedia.org/wiki/Heterotrophic heterotrophic] bacteria eat other microbes, including cyanobacteria. [[#References|[4.16]]] | ||
====Metabolic Adaptations==== | ====Metabolic Adaptations==== | ||
A few microorganisms, including bacteria, protozoa, lichens and algae, have stress-resistant, or dormant phases to help them endure subzero temperatures and an intermittent water supply, but these strategies can slow, or halt, metabolism and reproduction. In dormant phase, bacteria can alter their DNA to make it more resistant to UV damage. This is especially important because the hole in the ozone layer over Antarctica admits large amounts of solar radiation. | A few microorganisms, including bacteria, [http://en.wikipedia.org/wiki/Protozoa protozoa], lichens and algae, have stress-resistant, or dormant phases to help them endure subzero temperatures and an intermittent water supply, but these strategies can slow, or halt, [http://en.wikipedia.org/wiki/Metabolism metabolism] and [http://en.wikipedia.org/wiki/Reproduction reproduction]. In dormant phase, bacteria can alter their DNA to make it more resistant to UV damage. This is especially important because the hole in the ozone layer over Antarctica admits large amounts of solar radiation. [[#References|[4.13]]] | ||
Scientists have analyzed enzymes from psychrophilic (cold-loving) bacteria and compared them to functionally equivalent enzymes from microbes that live in warmer locations. A few modified proteins include: alcohol dehydrogenase, xylanase, α-amylase, β-lactamase, aspartate, citrate synthase, transcarbamylase, subtilisin, | Scientists have analyzed [http://en.wikipedia.org/wiki/Enzyme enzymes] from [http://en.wikipedia.org/wiki/Psychrophilic psychrophilic] (cold-loving) bacteria and compared them to functionally equivalent enzymes from microbes that live in warmer locations. A few modified proteins include: [http://en.wikipedia.org/wiki/Alcohol_dehydrogenase alcohol dehydrogenase], [http://en.wikipedia.org/wiki/Xylanase xylanase], [http://en.wikipedia.org/wiki/Α-amylase α-amylase], [http://en.wikipedia.org/wiki/Β-lactamase β-lactamase], [http://en.wikipedia.org/wiki/Aspartate aspartate], [http://en.wikipedia.org/wiki/Citrate_synthase citrate synthase], transcarbamylase, [http://en.wikipedia.org/wiki/Subtilisin subtilisin], Ca<sup>2+</sup>–Zn<sup>2+</sup> protease, [http://en.wikipedia.org/wiki/Malate_dehydrogenase malate dehydrogenase] and [http://en.wikipedia.org/wiki/Triose_phosphate_isomerase triose phosphate isomerase]. These enzymes have higher activity in the cold (0-30°C) than their [http://en.wikipedia.org/wiki/Mesophile mesophilic] counterparts, but at warmer temperatures the cold enzymes [http://en.wikipedia.org/wiki/Denaturation_(biochemistry) denature]. | ||
Cold-adapted enzymes have a more flexible structure than those from thermophiles, or mesophiles. This flexibility is probably due to weaker bonds within subunits, fewer ion interactions, weaker intramolecular bonds, stronger solvent interactions, higher amounts of glycyl residues, and lower numbers of prolyl and arginyl residues. The extreme cold makes normal enzyme activity impossible because with normal-strength bonds the structures become too rigid to function as catalysts. The weaker bonds allow the microbe to overcome this problem. | Cold-adapted enzymes have a more flexible structure than those from thermophiles, or mesophiles. This flexibility is probably due to weaker bonds within subunits, fewer ion interactions, weaker intramolecular bonds, stronger solvent interactions, higher amounts of glycyl residues, and lower numbers of prolyl and arginyl residues. The extreme cold makes normal enzyme activity impossible because with normal-strength bonds the structures become too rigid to function as catalysts. The weaker bonds allow the microbe to overcome this problem. [[#References|[4.17]]] | ||
In periods of frigid cold, most psychrophilic bacteria are in stationary phase (survival phase), rather than growth phase. Photosynthetic activity in Antarctic lichens has been observed at temperatures as low as -17° C | Increasing [http://en.wikipedia.org/wiki/Membrane_fluidity membrane fluidity] is another method microbes have developed to adapt to the cold. They have done this by increasing the proportion of unsaturated and polyunsaturated fatty acids. Unsaturated fatty acids have double-bond; double-bonds give fatty acids a lower melting point and viscosity than fatty acids with all single bonds. [[#References|[4.25]]] | ||
In periods of frigid cold, most psychrophilic bacteria are in stationary phase (survival phase), rather than growth phase. Photosynthetic activity in Antarctic lichens has been observed at temperatures as low as -17° C. [[#References|[4.18]]] | |||
====Environmental Interaction==== | ====Environmental Interaction==== | ||
Some cyanobacteria fix nitrogen, though, it is not yet known whether the species found in Antarctica do this. | Some cyanobacteria [http://en.wikipedia.org/wiki/Nitrogen_fixing fix nitrogen], though, it is not yet known whether the species found in Antarctica do this. [[#References|[4.12]]] | ||
Little is known about endolithic microbial communities because not much research has been performed. They are thought to take part in the weathering of rocks and the cycling of elements and nutrients. | Little is known about endolithic microbial communities because not much research has been performed. They are thought to take part in the weathering of rocks and the [http://en.wikipedia.org/wiki/Nutrient_cycle cycling of elements and nutrients]. [[#References|[4.10]]] | ||
Lichen attach to the surfaces of rocks and extract minerals from them. | Lichen attach to the surfaces of rocks and extract minerals from them. [[#References|[4.14]]] | ||
Though oil spills have proven to lower diversity, some species of bacteria can degrade hydrocarbons, thus removing the waste. Some of those that do belong to the phylums Pseudomonas, Sphingomonas, and Rhodococcus. | Though oil spills have proven to lower diversity, some species of bacteria can degrade hydrocarbons, thus removing the waste. Some of those that do belong to the phylums Pseudomonas, Sphingomonas, and Rhodococcus. [[#References|[4.26]]] | ||
[[# | [[#Slush |Back to top]] | ||
==Sea Ice== | ==Sea Ice== | ||
| Line 270: | Line 275: | ||
====Location==== | ====Location==== | ||
Antarctic sea ice surrounds the southernmost continent year around. Due to the 3.5% salinity of the ocean water, sea ice forms at a lower temperature than freshwater: -1.8 °C (28.8 °F) compared to 0 °C (32 °F) [5.1]. | Antarctic sea ice surrounds the southernmost continent year around. Due to the 3.5% salinity of the ocean water, sea ice forms at a lower temperature than freshwater: -1.8 °C (28.8 °F) compared to 0 °C (32 °F) [[#Referrences|[5.1]]]. | ||
The amount of sea ice is consantly changing the size of Antarctica throughout the various seasons. The total land mass of Antarctica without ice is 280,000 km² (108,108.6 sq mi). However, at the peak of winter, Antarctica, when combined with sea ice, reaches a total area of 13,720,000 km² (5,297,321.6 sq mi), which is nearly 1.5 times the size of the United States [5.2]. | The amount of sea ice is consantly changing the size of Antarctica throughout the various seasons. The total land mass of Antarctica without ice is 280,000 km² (108,108.6 sq mi). However, at the peak of winter, Antarctica, when combined with sea ice, reaches a total area of 13,720,000 km² (5,297,321.6 sq mi), which is nearly 1.5 times the size of the United States [[#References|[5.2]]]. | ||
Sea ice is often confused with icebergs; the other major form of ice surrounding Antarctica. Icebergs are made from either broken-off portions of glaciers or ice shelves. In comparison, icebergs are produced from precipitation and are therefore freshwater, while sea ice is produced from freezing seawater. | Sea ice is often confused with icebergs; the other major form of ice surrounding Antarctica. Icebergs are made from either broken-off portions of glaciers or ice shelves. In comparison, icebergs are produced from precipitation and are therefore freshwater, while sea ice is produced from freezing seawater. | ||
====Physical Conditions==== | ====Physical Conditions==== | ||
'''Temperature''' | |||
The temperature of the Southern Ocean fluctuates throughout the seasons. In the northerly regions of Antarctica, Signy Island for example, the ocean temperature ranges between -1.8°C in the winter and +1.0°C in the summer [5.3]. However, in more southerly areas, such as McMurdo Sound, the ocean temperature ranges between -2.0°C and -1.7°C annually [5.3]. In comparing the total ocean temperature throughout the Antarctic region, the water temperature rarely fluctuates more than 3°C “making this one of the most thermally stable environments on earth” [5.3]. According to the studies of Lloyd S Peck [5.3], the ocean temperature in the Antarctic region has been low and stable for at least 10 million years. | The temperature of the Southern Ocean fluctuates throughout the seasons. In the northerly regions of Antarctica, Signy Island for example, the ocean temperature ranges between -1.8°C in the winter and +1.0°C in the summer [[#References|[5.3]]]. However, in more southerly areas, such as McMurdo Sound, the ocean temperature ranges between -2.0°C and -1.7°C annually [[#References|[5.3]]]. In comparing the total ocean temperature throughout the Antarctic region, the water temperature rarely fluctuates more than 3°C “making this one of the most thermally stable environments on earth” [[#References|[5.3]]]. According to the studies of Lloyd S Peck [[#References|[5.3]]], the ocean temperature in the Antarctic region has been low and stable for at least 10 million years. | ||
'''Light''' | |||
The amount of light that reaches sea ice varies depending on the time of year. Due to the high latitude, seasonal light “varies between no direct sunlight... to 24 h of direct sunlight” [5.3]. Varying amounts of light causes an enormous shift in sea ice formation. During winter, the light source is limited and decreases the air temperature. This contributes to an overall radiative loss, which causes sea ice to form at a rapid rate. The radiative loss is countered in the summer when the radiative value equals or surpasses that of tropical regions. These opposing radiative values contribute to the annual growth and decline of 10-15 million km<sup>2</sup> of sea ice [5.3]. | The amount of light that reaches sea ice varies depending on the time of year. Due to the high latitude, seasonal light “varies between no direct sunlight... to 24 h of direct sunlight” [[#References|[5.3]]]. Varying amounts of light causes an enormous shift in sea ice formation. During winter, the light source is limited and decreases the air temperature. This contributes to an overall radiative loss, which causes sea ice to form at a rapid rate. The radiative loss is countered in the summer when the radiative value equals or surpasses that of tropical regions. These opposing radiative values contribute to the annual growth and decline of 10-15 million km<sup>2</sup> of sea ice [[#References|[5.3]]]. | ||
During the summer, phototropic microorganisms that are able to survive atop of the sea ice are exposed to harsh temperatures, high amounts of light, and an increasing amount of UV radiation. Phototropic microorganisms under the sea ice have a more stable environment, yet only have access to less than 1% of light. In contrast, when the Antarctic is between solar light cycles during the winter months, phototropic microorganisms must utilize their evolutionary traits to survive nearly 5 months without light [5.4]. | During the summer, phototropic microorganisms that are able to survive atop of the sea ice are exposed to harsh temperatures, high amounts of light, and an increasing amount of UV radiation. Phototropic microorganisms under the sea ice have a more stable environment, yet only have access to less than 1% of light. In contrast, when the Antarctic is between solar light cycles during the winter months, phototropic microorganisms must utilize their evolutionary traits to survive nearly 5 months without light [[#References|[5.4]]]. | ||
'''Weather''' | |||
Antarctica is the coldest and windiest place on Earth. The climate that surrounds Antarctica’s sea ice is some of the most unforgiving on Earth, which is only rivaled by Antarctica’s mountainous terrain. In the winter, the lowest recorded temperature is -89.2°C (-128.6°F) and the highest recorded temperature during summer is +15°C (+59°F). On average the summer temperature is -27.5°C (-17.5°F), contrary to -60°C (-76°F) during the winter. | Antarctica is the coldest and windiest place on Earth. The climate that surrounds Antarctica’s sea ice is some of the most unforgiving on Earth, which is only rivaled by Antarctica’s mountainous terrain. In the winter, the lowest recorded temperature is -89.2°C (-128.6°F) and the highest recorded temperature during summer is +15°C (+59°F). On average the summer temperature is -27.5°C (-17.5°F), contrary to -60°C (-76°F) during the winter. | ||
Wind also affects the extreme temperatures of Antarctica. The average inland wind speed is 12 mph. However, on the coast where sea ice is formed, the average wind speed is 198 mph. These high winds in combination with low temperatures make Antarctica the driest desert in the world. These desert conditions arise due to the average humidity revolving around 0.03%, which causes the average precipitation to be less than 1” annually [5.5]. | Wind also affects the extreme temperatures of Antarctica. The average inland wind speed is 12 mph. However, on the coast where sea ice is formed, the average wind speed is 198 mph. These high winds in combination with low temperatures make Antarctica the driest desert in the world. These desert conditions arise due to the average humidity revolving around 0.03%, which causes the average precipitation to be less than 1” annually [[#References|[5.5]]]. | ||
====Changing Conditions==== | ====Changing Conditions==== | ||
Observations of Antarctic conditions have only been recorded for the last 150 years. However, due to limited resources and technology, constant climate monitoring was not possible until the 1950’s [5.5]. Currently, many bases are located on the continent and are equipped with advanced weather recording devices to observe conditions year-round. In addition, satellite technology has been utilized to survey the environment and weather patterns. | Observations of Antarctic conditions have only been recorded for the last 150 years. However, due to limited resources and technology, constant climate monitoring was not possible until the 1950’s [[#References|[5.5]]]. Currently, many bases are located on the continent and are equipped with advanced weather recording devices to observe conditions year-round. In addition, satellite technology has been utilized to survey the environment and weather patterns. | ||
Above the Antarctic sea ice conditions are constantly changing. Frequent changes in weather alter the landscape of the sea ice consistently. Throughout the year, with the combination of unpredictable temperature changes and hurricane force winds, temporary sea ice fluctuates by 10-15 million km<sup>2</sup> [5.3]. However, as previously stated, the ocean’s temperature rarely alters more then 3°C and has done so for more than 10 million years [5.3]. | Above the Antarctic sea ice conditions are constantly changing. Frequent changes in weather alter the landscape of the sea ice consistently. Throughout the year, with the combination of unpredictable temperature changes and hurricane force winds, temporary sea ice fluctuates by 10-15 million km<sup>2</sup> [[#References|[5.3]]]. However, as previously stated, the ocean’s temperature rarely alters more then 3°C and has done so for more than 10 million years [[#References|[5.3]]]. | ||
===Inhabitants=== | ===Inhabitants=== | ||
====Microbes==== | ====Microbes==== | ||
| Line 301: | Line 307: | ||
The Southern Ocean has a very low temperature and a high concentration of salt, yet microbial life is able to thrive in these extreme conditions. Microbes that are able to live within the freezing temperatures are known as psychrophilies. Some microbes that live within the sea ice are also halophilic because they are able to live in extremely salty conditions. | The Southern Ocean has a very low temperature and a high concentration of salt, yet microbial life is able to thrive in these extreme conditions. Microbes that are able to live within the freezing temperatures are known as psychrophilies. Some microbes that live within the sea ice are also halophilic because they are able to live in extremely salty conditions. | ||
According to Nichols [5.1]: | According to Nichols [[#References|[5.1]]]: | ||
“The sea ice produces highly variable microenvironments in terms of temperature, salinity, nutrient concentration, and light intensities within the columns, which may be as thick as 2 m. Salinity in sea ice brine can range from near that of freshwater to >15% at the ice-seawater interface. Temperatures can range from 0°C to -35°C. Sea ice is thus one of the coldest habitats on earth for marine life.” | “The sea ice produces highly variable microenvironments in terms of temperature, salinity, nutrient concentration, and light intensities within the columns, which may be as thick as 2 m. Salinity in sea ice brine can range from near that of freshwater to >15% at the ice-seawater interface. Temperatures can range from 0°C to -35°C. Sea ice is thus one of the coldest habitats on earth for marine life.” | ||
Some psychrophilic bacteria have evolved to contain gas vacuoles; this allows them buoyancy to float at a certain level in order to regulate their position in vertically stratified water columns and adapt to their environment [5.6]. These gas vacuole-containing bacteria fall into four main classes: alpha, beta, and gamma Proteobacteria and the Flavobacteria-Cytophaga group. The gas vacuoles give sea ice bacteria an energy-maximizing advantage due to the generally higher enrichment in sea ice compared to other bacteria in open and underlying seawater, which allows bacterial cell biovolumes to grow 5 to 10 times larger [5.7]. | Some psychrophilic bacteria have evolved to contain gas vacuoles; this allows them buoyancy to float at a certain level in order to regulate their position in vertically stratified water columns and adapt to their environment [[#References|[5.6]]]. These gas vacuole-containing bacteria fall into four main classes: alpha, beta, and gamma Proteobacteria and the Flavobacteria-Cytophaga group. The gas vacuoles give sea ice bacteria an energy-maximizing advantage due to the generally higher enrichment in sea ice compared to other bacteria in open and underlying seawater, which allows bacterial cell biovolumes to grow 5 to 10 times larger [[#References|[5.7]]]. | ||
====Other Organisms==== | ====Other Organisms==== | ||
Phytoplankton living in the Southern Ocean are responsible for it being one of the most productive oceans in the world [5.8]. Phytoplanktons are photosynthetic organisms. During the transition from winter to spring, the recession of Antarctic sea ice promotes massive phytoplankton blooms. Due to their sudden growth, the normal chlorophyll level rises from less than 5 μg chlorophyll a/liter, to an amazing 1,000 μg chlorophyll a/liter, which not only helps produce half of the worlds oxygen supply but also perpetuates one of the largest links in the Southern Ocean’s food chain [5.4]. | Phytoplankton living in the Southern Ocean are responsible for it being one of the most productive oceans in the world [[#References|[5.8]]]. Phytoplanktons are photosynthetic organisms. During the transition from winter to spring, the recession of Antarctic sea ice promotes massive phytoplankton blooms. Due to their sudden growth, the normal chlorophyll level rises from less than 5 μg chlorophyll a/liter, to an amazing 1,000 μg chlorophyll a/liter, which not only helps produce half of the worlds oxygen supply but also perpetuates one of the largest links in the Southern Ocean’s food chain [[#References|[5.4]]]. | ||
====Microbe Interaction==== | ====Microbe Interaction==== | ||
Phytoplanktons produce much of the atmosphere’s oxygen by consuming | Phytoplanktons produce much of the atmosphere’s oxygen by consuming CO<sub>2</sub>. Two of the essential nutrients of phytoplankton are nitrate and phosphate. During occasionally large phytoplankton blooms, or in areas of large phytoplankton accumulation, surface nitrate and phosphate may be nearly depleted [[#References|[5.9]]]. When the resources are depleted the phytoplankton die off, creating an accumulation of slow-to-degrade dissolved organic matter (DOM), which fuels bacteria production through the winter [[#References|[5.10]]]. Utilization of DOM by bacteria creates the byproducts nitrogen and phosphate, which contributes to the renutrification of the ocean. | ||
====Metabolic Adaptation==== | ====Metabolic Adaptation==== | ||
Both bacteria and phytoplankton are psychrophilic microbes. Due to the low temperature, these organisms have adapted and evolved. In order to grow and function, they did as follows: “reduced enzyme activity; decreased membrane fluidity; altered transport of nutrients and waste products; decreased rates of transcription, translation and cell division; protein cold-denaturation; inappropriate protein folding; and intracellular ice formation” [5.11]. | Both bacteria and phytoplankton are psychrophilic microbes. Due to the low temperature, these organisms have adapted and evolved. In order to grow and function, they did as follows: “reduced enzyme activity; decreased membrane fluidity; altered transport of nutrients and waste products; decreased rates of transcription, translation and cell division; protein cold-denaturation; inappropriate protein folding; and intracellular ice formation” [[#References|[5.11]]]. | ||
[[# | [[#Slush |Back to top]] | ||
==Freshwater Ice== | ==Freshwater Ice== | ||
| Line 329: | Line 335: | ||
The Antarctic is one of Earth's extreme places. Many different species of microbes and flora are unique to Antarctica, suggesting many years of evolutionary isolation. Due to the extreme conditions faced by the multitude of microorganisms inhabiting the continent, many have evolved remarkable biochemical, physiological and behavioral adaptations, the study of which is leading to the discovery of useful chemicals and genes. These organisms live in an environment that is rapidly changing due to the effects of global warming. Antarctica is a unique natural laboratory for investigating the effects of environmental changes on the structure and function of biological communities and their genetic makeup. | The Antarctic is one of Earth's extreme places. Many different species of microbes and flora are unique to Antarctica, suggesting many years of evolutionary isolation. Due to the extreme conditions faced by the multitude of microorganisms inhabiting the continent, many have evolved remarkable biochemical, physiological and behavioral adaptations, the study of which is leading to the discovery of useful chemicals and genes. These organisms live in an environment that is rapidly changing due to the effects of global warming. Antarctica is a unique natural laboratory for investigating the effects of environmental changes on the structure and function of biological communities and their genetic makeup. | ||
====Physical Conditions | ====Physical Conditions==== | ||
Approximately 61 percent of all fresh water on the Earth is held in the Antarctic ice sheet, an amount equivalent to 70 m of water in the world's oceans. In East Antarctica, the ice sheet rests on a major landmass, but in West Antarctica the bed can extend to more than 2,500 m below sea level. The land would be seabed if the ice sheet were not there. | Approximately 61 percent of all fresh water on the Earth is held in the Antarctic ice sheet, an amount equivalent to 70 m of water in the world's oceans. In East Antarctica, the ice sheet rests on a major landmass, but in West Antarctica the bed can extend to more than 2,500 m below sea level. The land would be seabed if the ice sheet were not there. | ||
Temperatures in the Antarctic can be intense and fluctuate from about -85°C in austral winter, while in summer the temperature can warm to about -13°C (mean monthly air temperature in December is -26°C). Microbes, in particular bacteria, have been cultured from samples taken from Antarctic ice cores, and deep cores from the accreted ice above subglacial Lake Vostok and have revealed a high diversity of species that were reported to be metabolically active when warmed to 3°C [1]. | Temperatures in the Antarctic can be intense and fluctuate from about -85°C in austral winter, while in summer the temperature can warm to about -13°C (mean monthly air temperature in December is -26°C). Microbes, in particular bacteria, have been cultured from samples taken from Antarctic ice cores, and deep cores from the accreted ice above subglacial Lake Vostok and have revealed a high diversity of species that were reported to be metabolically active when warmed to 3°C [[#References |[6.1]]]. | ||
====Affect of Adjacent Communities==== | ====Affect of Adjacent Communities==== | ||
Ancient Antarctic microbial communities have persisted for many years, and throughout their course some have evolved traits to help them survive in complete isolation. Some microbial communities such as those that have evolved to sustain themselves on their own are not affected by adjacent communities, while organisms such as phytoplankton rely on the collective process cycling compounds such as nitrogen and gathering light and nutrients to sustain an existence from the environment. | |||
====Changing Conditions==== | ====Changing Conditions==== | ||
Limnological parameters including: water temperature, light availability, turbidity, and chlorophyll a concentration vary with the seasons. Water is in a liquid phase throughout the year, with temperatures ranging from 0 to 10°C [[#References |[6.2]]] | |||
Even though the Antarctic benefits from a continuous amount of sunlight during the summer months, the long and dark winter months result in the lowest annual levels of photosynthetically active radiation (PAR) at the surface of the Earth [[#Reference|[6.3]]]. Not only are polar lakes greatly restricted from sunlight during the winter months, but the presence of ice and snow that cover them further make it difficult for light to reach the photosynthesizing organisms in the water. Antarctic lake environments are considered as extremely low productive ecosystems because of their oligotrophic situation and the low temperatures to which they are constantly exposed. Therefore, the theory is that the growth stage of phototrophs in continental Antarctic lakes (phytoplankton and benthic algae are the main primary producers in these lakes) can take place only during the summer [[#References |[6.4]]]. | |||
The impact of increasing solar ultraviolet-B radiation (UVB, 280-320 nm) on aquatic ecosystems has been of greatest concern in the southern polar region where the annual depletion of stratospheric ozone now extends from spring into late summer [[#References |[6.5]]]. There have been quite a few studies done in regards to the penetration and potential effects of UVB in the Southern Ocean but not much is known about non-marine ecosystems such as lakes, etc. Antarctic lakes and streams have unique microbial ecosystems and contain a species-poor community structure that is limited by extreme isolation and the harsh continental environment [[#References |[6.6]]]. These communities must now contend with the additional stress of increasing short-wave ultraviolet radiation. In many lakes in the temperate zone, the aquatic biota are protected from UVB (280-320 nm) and to a lesser extent UVA (320-400 nm) by the presence of chromophoric dissolved organic matter (CDOM). These materials are composed of aromatic humic and fulvic acids brought in from vegetation and leaf litter in the surrounding catchment [[#References |[6.7]]]. | |||
===Inhabitants=== | ===Inhabitants=== | ||
Prokaryotes are abundant and active in polar environments [[#References |[6.11]]]. Antarctic lakes are particularly interesting in this respect because they are exclusively microbial ecosystems [[#References |[6.12]]]. Freshwater lakes occur through much of Antarctica and are characterized by short food chains dominated by microbes. | |||
====Microbes and Microbe Interaction==== | |||
Comparatively few studies have been made of continental freshwater lakes until recently, with the main emphasis being on the less extreme maritime Antarctic lakes. Information on seasonal and spatial patterns of microbial activity for freshwater lakes demonstrates rapid changes in community composition at certain times of year despite constant low temperatures. Benthic communities of cyanobacteria and bacteria are a feature of most lakes and are involved in a wide range of geochemical cycling [[#References |[6.13]]]. | |||
Lakes in the McMurdo Dry Valleys of Antarctica are characterized by a permanent ice cover and little or no anthropogenic influence. The sequencing of 16S rRNA genes of randomly selected representative bacterial cultures from fresh surface water of Lake Fryxell and the hypersaline, suboxic bottom water from Lake Bonney revealed that the corresponding isolates belonged to the Alphaproteobacteria, Betaproteobacteria, Bacteroidetes, and Actinobacteria. Phylogenetic analysis of the sequences showed that the vast majority of the isolates were not closely related to previously described species [[#References |[6.14]]]. | |||
The invertebrate fauna of many Antarctic ice-free areas, even those close to permanent research stations, are poorly known. Nematodes from freshwater and saline, marine-derived lakes of the Vestfold Hills, East Antarctica exist. The freshwater lakes contain the widespread East Antarctic endemic species, Plectus frigophilus. The saline lakes were inhabited by two recently described species, Halomonhystera halophila and Halomonhystera continentalis, and by a new species, Hypodontolaimus antarcticus. The nematode fauna of Antarctica now consists of 54 named species, 22 of which are found in East Antarctica [[#References |[6.15]]]. | |||
====Environmental Interaction==== | ====Environmental Interaction==== | ||
In order for ecosystems to sustain themselves, the organisms that inhabit them must possess attributes that are adequate and diverse enough to provide all of the basic pathways that permit the processes of ecosystems to function. These processes include: nutrient cycling, photosynthesis, the range of catabolic pathways (aerobic and anaerobic), nitrogen fixation, the nitrogen and sulphur cycles and trace element metabolism. The nitrogen cycle is an important microbially mediated metabolic pathway present in Antarctic lakes and other aquatic systems [[#References |[6.8]]]. Nitrogen uptake and nitrogen fixation have been characterized in inland Antarctic waters and at similar rates to other ecosystems at lower altitudes. However, the unique features of Antarctic ecosystems stem from the extreme and variable physical conditions under which these processes such as the nitrogen cycle operate [[#References |[6.8]]]. | |||
Antarctic lakes in the McMurdo Dry Valleys contain a complex food web that is driven both by ongoing organic carbon production and carbon left as a legacy of past events. Production of organic carbon by autotrophic organisms and the heterotrophic transformations that follow initiate biogeochemical reactions that influence diversity and abundance of species in all ecosystems. The timing and magnitude of organic carbon production is particularly critical in desert ecosystems, where extreme temperatures and a lack of liquid water limits the period when biological activity can persist. Another stress faced on the system is the seasonal change of available sunlight. It is now clear that the presence of liquid water in the McMurdo Dry Valleys produces a cascade of tightly coupled events that ultimately leads to the biological production and cycling of organic carbon and related elements [[#References |[6.9]]]. The lack of water and the sensitive balance between gains and losses of organic carbon make the McMurdo Dry Valleys ecosystem one of the most delicate indicators of environmental change on the planet. Antarctica’s extreme conditions, in which certain chemical, physical, and biological properties are exaggerated make Antarctic lakes ideal experimental systems that offer potential insights into ecosystem structure and function that are not obvious in less extreme systems [[#References |[6.9]]]. | |||
====Metabolic Adaptation==== | ====Metabolic Adaptation==== | ||
Analysis of metals in Lake Vanda, a permanently ice-covered, stratified Antarctic lake, suggest the importance of particulate manganese oxides in the scavenging, transport, and release of metals. Lake Vanda is filled with manganese-reducing bacteria and based on phylogenetic analysis of the 16S rRNA gene sequence, many manganese reducers are part of the genus Carnobacterium. Whereas anaerobes use manganese oxides as their terminal electron acceptors in cellular respiration, isolates of Carnobacterium were shown to reduce Mn(IV) by means of a diffusible compound under oxic conditions [[#References |[6.16]]]. It was shown that the release of adsorbed trace metals accompanying the solubilization of manganese oxides most likely supply populations of Carnobacterium with a source of nutrients, where the environment is very oligotrophic [[#References |[6.16]]] | |||
From studies at Lake Hoare, South Victoria Land, Antarctica, exposure of benthic and planktonic microbes to Ultraviolet radiation (UVR) encountered immediately beneath the ice is unlikely to inhibit microbial metabolism [[#References |[6.17]]]. Even though waters of oligotrophic antarctic lakes are very transparent to UVR, the thick, high scattering and optically dense ice covers on many of these lakes offers organisms a degree of protection largely unavailable in temperate and tropical systems [[#References |[6.17]]]. With the effects of global warming taking effect, thinning or complete loss of these overlying ice covers is likely to have major consequences for the structure of antarctic lake microbial communities. | |||
[[# | |||
[[#Slush |Back to top]] | |||
==Current Research== | ==Current Research== | ||
===Primary Research=== | |||
Ongoing research is constantly being done in order to ascertain how psychophillic bacteria are able to function and survive in the cold harsh Antarctic climate. One such adaptation is the presence of antifreeze proteins (AFPs) which allow microbes to live in subzero temperatures. Furthermore, each bacterium may contain different types of AFPs as this group of proteins is very diverse. Only a handful of bacteria are known to have AFPs which include Pseudomonas putida (Sun et al., 1995; Xu et al., 1998; Kawahara et al., 2001), Micrococcus cryophilus, Rhodococcus erythropolis (Duman & Olsen, 1993), Marinomonas protea (Mills, 1999) and a Moraxella species (Yamashita et al., 2002). Scientists hypothesize that the difference between each AFPs are due to the environments the bacterium resides in which in turn shape the evolution of these proteins. As a result, measuring AFP activity of different organisms using the available techniques may not be sufficient. One study takes a look at the differences in AFPs in different Antarctic environments such as freshwater and seawater which range from average freshwater salinity to 240% salinity and then attempts to develop new assays in order to measure AFP activity in each unique organism. One assay developed, called the HTAP assay measures light refraction in the sample. Samples with no AFP activity will appear to be transparent while samples with AFP activity will appear opaque due to the small dense crystals AFP proteins help create. Another assay, the splat assay which uses crossed polarized microscopy, was used to verify positive AFP activity results from the HTAP assay. This was necessary because color pigmentation from other sources, other than the ice itself, may skew the results of the light based HTAP assay. [[#References |[1.8]]] | |||
Raymond et al. discuss the possibility of specific adaptations for survival within ice of microorganisms. The Researchers hypothesized that a particular type of ice-binding protein should be present in microbes found in the samples of the ice cores, as a similar adaptation can be found in bacteria which reside in antifreeze. The protein in question is one that specifically alters the structure of the ice crystals around the organism allowing it to survive. | |||
Potential contaminants were removed from the exterior of the ice cores as per protocol. Ice samples were melted and subsequently incubated at 4°C. Following incubation the isolates were grown on different media, either R2 or agar-solidified. Fourteen species were isolated (mentioned above). An ice-pitting assay was preformed to determine whether any of these microbes held the ability in question. Only one bacterium showed activity and caused hexagonal pitting. A series of comparisons was undertaken to determine the bacterium's place within the phylogenetic tree. Though it produced ice-binding proteins (IBP) similar to other bacteria living in icy environments the proteins were distinct enough for the chance of the bacterium to be a contaminant to be very small.[[#References |[2.7]]] | |||
[[ | Biotech and pharmaceutical companies are becoming increasingly interested in compounds extracted from Antarctic life forms These organisms live in harsh frozen conditions and have developed complex mechanisms to deal with the low temperature, lack of nutrients, and other difficulties of living in such an extreme environment. Companies that develop industrial processes that keep food or medicines fresh while at a low temperature are currently conducting research on unique enzymes found in Antarctic organisms that can aid the process of chilling without killing. As of 2004, 154 patents have been obtained for compounds naturally found in the Antarctic, including one for a glycoprotein found in a bacterium that has been shown to heal wounds and skin and benefit hair and nails [[#References |[3.6]]]. | ||
The problem with this research is that it is difficult to know how much is purely for scientific purposes and how much is for financial gain. Companies looking for novel compounds to turn into drugs for profit may cause environmental damage or exploit the resources found on the greatly undisturbed continent. Fortunately, many Antarctic researchers have expressed this concern and are trying to put regulations in place to prevent abuse of resources [[#References |[3.6]]]. | |||
P. Buford Price from the Physics Department, University of California, Berkeley, CA and Todd Sowers from the Department of Geosciences, Pennsylvania State University, University Park, PA. performed studies on the metabolic rates in microbes at very low temperatures. Price and Sowers compared many different substances to determine different metabolic rates of microbial communities. Their findings demonstrated that there is no minimum temperature for metabolism [[#References|[5.12]]]. In fact, Price and Sowers show that microbes in ice have similar metabolic rates to other substances at the same temperature. Therefore, the data confirms that liquid water inside ice is available for metabolism [[#References|[5.12]]]. | |||
There is also rising interest in bacteria that are capable of cleaning up oil spills and other pollution in Antarctica. Only cold-adapted organisms would be able to take part in this bioremediation.[[#References |[4.23]]] | |||
Hydrocarbon Contamination and Biodegradation Within the Permanent Ice Cover of Lake Fryxell, Antarctica: | |||
Psychrophilic enzymes are of commercial interest because of their ability to carry out reactions at low temperatures and have potential utility in bioremediation. Another goal is to find organisms that can safely degrade toxic organic contaminants (for example, petroleum) in the cold. Artic environments are particularly sensitive to pollutions because contaminants are slow to degrade in the freezing temperatures. | |||
During the summer of 2003, a helicopter crashed onto the permanent ice cover of Lake Fryxell contaminating the surface with several hundred liters of jet fuel (JP8). Researchers examined the influence of this spill on the microbial community that resides within the ice cover [[#References |[6.10]]]. | |||
The researchers wanted to test whether there are hydrocarbon degrading organisms present in the natural lake ice community. Their experiments measured the degradation of JP8 jet fuel, as well as fractions of this fuel including naphthalene (aromatic) and nonane (C9 alkane)[[#References |[6.10]]]. Studies have shown enhanced degradation of hydrocarbons in Antarctic soils with the addition of N and P, therefore our experimental treatments were run with and without additional N and P. | |||
These experiments demonstarted that the native ice community found in Lake Fryxell is capable of degrading JP8 jet fuel and fractions of the fuel. Respirometry experiments showed that addition of N and P increased the rate of degradation, this may be due to the fact that this environment is limited in these nutrients. A change in community and diversity was observed in both the TTGE analysis and culturing methods suggesting that the hydrocarbon spill changed the community structure of the lake ice [[#References |[6.10]]]. | |||
[[Image:Mars.jpg|left|frame|Mars with Polar Ice Cap]] | |||
Researchers continue to discover microbes deeper and deeper within the soil of the Antarctic Dry Valleys. Since Antarctic conditions below the soil or ice are so close to the subzero, parched, high UV radiation, anaerobic, icy environment of polar Mars, many believe that these findings give hope of uncovering microbial life, or fossilized remains of life, on our neighboring planet.[[#References |[4.19]]] Furthermore, studies have uncovered evidence that suggests that the global climate on early Earth and Mars were comparable. About 3.5 billion years ago, Mars is believed to have had large amounts of liquid water and temperate weather. The idea is if the at one time or another the planets had analogous physical conditions, and those conditions favored the emergence of life on Earth, it is very possible that life also appeared on Mars. Now that scientists have found life on Earth that can withstand the current climate of Mars, they retain the hope that if life did evolve, some organisms may still be alive. The scientists have also laid out various approaches for determining the presence of life on Mars: direct methods like culturing, observation of cell structure, measurements of activity and chemical markers; and indirect methods such as searching for fossils, coal, and “bioweathering,” and testing the composition of the atmosphere for oxygen and other gases. [[#References |[4.28]]] | |||
===Antarctica Popular science=== | |||
Here are some links to Articles about Antarctica which have been published in popular science magazines. | Here are some links to Articles about Antarctica which have been published in popular science magazines. | ||
| Line 392: | Line 427: | ||
http://www.caml.aq/microbes/index.html - This is a Census of Marine Aquatic life. | http://www.caml.aq/microbes/index.html - This is a Census of Marine Aquatic life. | ||
===References=== | ==Conclusions== | ||
Antarctica is a niche inhabited by many diverse microbes. These single-celled organisms have adapted to the severe weather conditions in a variety of different ways. New species with unique attributes are unearthed very frequently. Some of the physical modifications are an increase in membrane fluidity, and a decrease in enzyme intramolecular bonds to make them more flexible. A few species have developed anti-freeze proteins to cope with the low temperatures, and ice binding proteins to prevent ice crystals from forming. Other bacteria use the acid veins in the ice to extract energy and, in order to survive, have developed positively-charged, or proton impermeable membranes, and proton-carrying enzymes. Water-dwelling bacteria have gas vacuoles that make them buoyant, and allow the suns rays to reach them for photosynthesis. When their surroundings become too challenging, microbes often slow their metabolism, halt reproduction, and can even form dormant spores to wait until conditions improve. Additionally, these organisms interact with their environment and are essential to the ecosystem of the Antarctic. Since many larger animals cannot tolerate the Antarctic cold, the microbial communities are the dominant forms of life. Phytoplankton consume C0<sub>2</sub> and produce much of our atmosphere's oxygen. Bacteria take part in all the nutrient cycles, especially the carbon and nitrogen cycles. A lot of research is currently being conducted to discover more about Antarctic microbe lifestyles. Many scientists want to utilize the exceptionally resilient Antarctic bacteria to remove environmental pollutants, such as fuel-spills and radioactive waste. Other bacteria have seemingly unrelated properties that may be useful in the medical field. Researchers have found bacteria that are so tough that they believe similar microbes may be living on Mars today. The special microorganisms of the Antarctic niche are promising discoveries in all aspects of science. | |||
==References== | |||
===Slush=== | |||
[1.1] World Meteroplogical Organization Information Site: http://www.dbcp.noaa.gov/seashelp/HtmlIceGlossary.htm#slush | |||
[1.2] National Geographic Data Center - National Satellite, Data, and Information Service. http://www.ngdc.noaa.gov/mgg/image/2minrelief.html (2006) | |||
[1.3] International Polar Foundation Information Site: http://www.sciencepoles.org/index.php?/home/ | |||
[1.4] Zhang D, Yong Y, Xin Y, Hong L, Zhou P, and Zhou Y. Colwellia polaris sp. Nov., a psychrotolerant bacterium isolated from Arctic sea ice. International Journal of Systematic and Evolutionary Microbiology (2008) Vol. 58 p. 1931-1934 | |||
[1.5] Raymond J, Fritsen C, Shen K. An Ice-binding Protein from an Antarctic Sea Ice Bacterium. FEMS Microbiology Ecology (2007) Vol. 61(2) p. 214-221 | |||
[1.6] Huston A, Haeggstrom J, Feller G. Cold Adaptation of Enzymes: Structural, Kinetic, and Microcalorimetic characterizations of an aminopeptidase from the Arctic psychrophile Colwellia and of Human Leuikotriene A4 Hydrolase. Biochemica et Biophysica Acta (2008) June 13. | |||
1 | [1.7] Huston A, Methe B, Deming J. Purification, Characterization, and Sequencing of an Extracellular Cold-Active Aminopetidase Produced by Marine Psychrophile Colwellia Starin 34H. American Society for Microbiology (2004) Vol. 70(2) p. 3321-3328 | ||
[1.8] Gilbert J, Hill P, Dodd C, Laybourn J. Demonstration of Antifreeze Protein Activity in Antarctica Bacteria. Microbiology (2004) Vol. 150 p. 171-180 | |||
===Lake Vostok=== | |||
[2.1] Inman M. Microbial ecology. The Dark and Mushy Side of a Frozen Continent. Science2007 Jul 6;317(5834):35-6. | |||
[2.2] Studinger M, Bella RE, Karnera GD, Tikkua AA, Holtb JW, Morseb DL, Richterb TG, Kempfb SD, Petersb ME, Blankenshipb DD, Sweeneyc RE, Rystromc VL. Ice cover, landscape setting, and geological framework of Lake Vostok, East Antarctica. Earth and Planetary Science Letters2003;205:195-210. | |||
[2.3] Lavire C, Normand P, Alekhina I, Bulat S, Prieur D, Birrien J-L, Fournier P, Hänni C, Petit J-R. Presence of Hydrogenophilus thermoluteolus DNA in accretion ice in the subglacial Lake Vostok, Antarctica, assessed using rrs, cbb and hox. Environmental Microbiology2006;8(12):2106-14. | |||
[2.4] Siegert MJ, Ellis-Evans JC, Tranter M, Mayer C, Petit JR, Salamatin A, Priscu JC. Physical, chemical and biological processes in Lake Vostok and other Antarctic subglacial lakes. Nature2001 Dec 6;414(6864):603-9. | |||
[2.5] Karl DM, Bird DF, Bjorkman K, Houlihan T, Shackelford R, Tupas L. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science1999 Dec 10;286(5447):2144-7. | |||
[2.6] Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN. Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environ Microbiol2001 Sep;3(9):570-7. | |||
[ | [2.7] Raymond JA, Christner BC, Schuster SC. A bacterial ice-binding protein from the Vostok ice core. Extremophiles2008 Jul 12. | ||
[2.8] Hayashi NR, Ishida T, Yokota A, Kodama T, Igarashi Y. Hydrogenophilus thermoluteolus gen. nov., sp. nov., a thermophilic, facultatively chemolithoautotrophic, hydrogen-oxidizing bacterium. International Journal of Systematic Bacteriology1999;49:783-6. | |||
===Ross Dependency=== | |||
[3.1] Dennett, Mark R., Mathot, Slyvie, Caron, David A., Smith, Walker O. Jr. and Lonsdale, Darcy J. “Abundance and distribution of phototrophic and heterotrophic nano- and microplankton in the southern Ross Sea”. Elsevier Science Ltd. 2001. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VGC-44HY14B-7&_user=4429&_coverDate=12%2F31%2F2001&_alid=779993472&_rdoc=1&_fmt=high&_orig=search&_cdi=6035&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=81b0af8d36e1d05829f4ab21a06faa93] | [3.1] Dennett, Mark R., Mathot, Slyvie, Caron, David A., Smith, Walker O. Jr. and Lonsdale, Darcy J. “Abundance and distribution of phototrophic and heterotrophic nano- and microplankton in the southern Ross Sea”. Elsevier Science Ltd. 2001. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VGC-44HY14B-7&_user=4429&_coverDate=12%2F31%2F2001&_alid=779993472&_rdoc=1&_fmt=high&_orig=search&_cdi=6035&_sort=d&_docanchor=&view=c&_ct=1&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=81b0af8d36e1d05829f4ab21a06faa93] | ||
| Line 434: | Line 486: | ||
[3.7] Price, P. Buford. “Life in Solid Ice”. Cornell University Library. 2 July 2005. p. 1-11. [http://arxiv.org/ftp/q-bio/papers/0507/0507004.pdf] | [3.7] Price, P. Buford. “Life in Solid Ice”. Cornell University Library. 2 July 2005. p. 1-11. [http://arxiv.org/ftp/q-bio/papers/0507/0507004.pdf] | ||
===Soil=== | |||
[4.1] "Antarctica - The World Factbook". United States Central Intelligence Agency (2007-03-08). Retrieved on 2007-03-14. | |||
[4.2] "Weather in the Antarctic". British Antarctic Survey. Retrieved on 2006-02-09. | |||
[4.3] Lyons, W.B et al. 1998. A Late Holocene desiccation of Lake Hoare and Lake Fryxell, McMurdo Dry Valleys, Antarctica. <i>Antarctic Science.</i> Vol 10 (3): pgs 247-256. | |||
[4.4] Foreman, C., B. Sattler, J. Mikucki, D. Porazinska and J.C. Priscu. 2007. Metabolic
Activity and Diversity of Cryoconites in the Taylor Valley, Antarctica. <i>Journal of
Geophysical Research - Biogeosciences.</i> Pgs 1-43. | |||
[4.5] Powers, Laura E., Diana W. Freckman, Mengchi Ho and Ross A. Virginia. (1995). McMurdo LTER: Soil properties associated with nematode distribution along an elevational transect in Taylor Valley, Antarctica. <i>Antarctic Journal of the United States.</i> 30 (5): 282-283. | |||
[4.6] Imperio T, Viti C, Marri L. (2008). Alicyclobacillus pohliae sp. nov., a thermophilic, endospore-forming bacterium isolated from geothermal soil of the north-west slope of Mount Melbourne (Antarctica). <i>Int J Syst Evol Microbiol.</i> 58 (Pt 1):221-5. | |||
[4.7] Wood, S. et al. (2008). Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. <i>The ISME Journal.</i> Vol 2, pgs 308–320. | |||
[4.8] Ho, Mengchi, Ross A. Virginia, Laura E. Powers, and Diana W. Freckman. (1995). Soil chemistry along a glacial chronosequence on Andrews Ridge, Taylor Valley. <i>Antarctic Journal of the United States.</i> Vol 30 (5): 310-311. | |||
[4.9] Hogg I.D., Craig Cary S., Convey P., Newsham K.K., O'Donnell A.G., Adams B.J., Aislabie J., (...), Wall D.H. (2006) Biotic interactions in Antarctic terrestrial ecosystems: Are they a factor? <i>Soil Biology and Biochemistry.</i> 38 (10), pp. 3035-3040. | |||
[4.10] de la Torre, J. R., B. M. Goebel, E. I. Friedmann, and N. R. Pace. (2003). Microbial diversity of cryptoendolithic communities from the McMurdo dry valleys, Antarctica. <i>Appl. Environ. Microbiol.</i> 69:3858-3867. [PubMed]. | |||
[4.11] Stackebrandt E, Rainey FA, and Ward-Rainey NL. (1997). Proposal for a new hierarchic classification system, Actinobacteria classis nov. <i>Int J Syst Bacteriol.</i> 47:479-491. | |||
[4.12] Mitsui, A. et al. (1986). Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. <i>Nature</i> 323, 720–722. | |||
( | [4.13] Hogg I.D., Craig Cary S., Convey P., Newsham K.K., O'Donnell A.G., Adams B.J., Aislabie J., (...), Wall D.H. (2006). Biotic interactions in Antarctic terrestrial ecosystems: Are they a factor? <i>Soil Biology and Biochemistry.</i> 38 (10), pp. 3035-3040. (same as (9)) | ||
( | [4.14] Rosmarie Honegger. (1991). FUNCTIONAL ASPECTS OF THE LICHEN SYMBIOSIS. <i>Annu. Rev. Plant Physiol. Plant Mol. Biol.</i> Vol 42. pgs 553-7. | ||
[4.15] Hunt, H. W. (1987). The detrital food web in shortgrass prairie. <i>Biology and Fertility of Soils.</i> Vol 3, pgs 1-2. | |||
[4.16] BOOKRAGS STAFF. "Heterotrophic Bacteria". (2005). August 23 2008. <http://www.bookrags.com/research/heterotrophic-bacteria-wmi/>. | |||
[[4.17] Gerday C., Aittaleb M., Bentahir M., Chessa J.-P., Claverie P., Collins T., D'Amico S., (...). (2000). Feller G. Cold-adapted enzymes: From fundamentals to biotechnology. <i>Trends in Biotechnology.</i> 18 (3), pp. 103-107. | |||
[4.18] Rivkina, E. I. Friedmann, C. P. McKay, and D. A. Gilichinsky, E. M. (2000). Metabolic Activity of Permafrost Bacteria below the Freezing Point. <i>Appl Environ Microbiol.</i> 66(8): 3230–3233. | |||
[4.19] Wynn-Williams D.D., Edwards H.G.M. (2000). Proximal Analysis of Regolith Habitats and Protective Biomolecules in Situ by Laser Raman Spectroscopy: Overview of Terrestrial Antarctic Habitats and Mars Analogs. <i>Icarus.</i> 144 (2), pp. 486-503. | |||
[4.20] Lancaster, N. (2002). Flux of Eolian Sediment in the McMurdo Dry Valleys, Antarctica: A Preliminary Assessment. <i>Arctic, Antarctic, and Alpine Research.</i> Vol. 34, No. 3 (Aug., 2002), pp. 318-323. | |||
[4.21] Fell J.W., Scorzetti G., Connell L., Craig S. (2006). Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with <5% soil moisture. <i>Soil Biology and Biochemistry.</i> 38 (10), pp. 3107-3119. | |||
[4.22] Wall D.H., Virginia R.A. (1999) Controls on soil biodiversity: Insights from extreme environments. <i>Applied Soil Ecology,</i> 13 (2), pp. 137-150. | |||
[4.23] Aislabie, J., McLeod, M., Fraser, R. (1998). Potential for biodegradation of hydrocarbons in soil from the Ross Dependency, Antarctica. <i>Applied Microbiology and Biotechnology.</i> Volume 49, Number 2. | |||
[4.24] STEVENS, M.I. and HOGG, I. D.. 2006. <i>Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a global indicator.</i> Vol 1. Pgs 1-13.. | |||
[4.25] D’Amico, Salvino, et al. (2006). Psychrophilic microorganisms: challenges for life. <i>European Molecular Biology Organization reports.</i> VOL 7 | NO 4, 385–389. | |||
[4.26] David J. Saul a , Jackie M. Aislabie b, *, Caroline E. Brown a , Lisa Harris a , Julia M. Foght c. (2006). Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. <i>FEMS Microbiology Ecology.</i> Volume 53, Issue 1, Pages 141-155. | |||
[4.27] Gupta, R. S., Johari, V. (1998). Signature Sequences in Diverse Proteins Provide Evidence of a Close Evolutionary Relationship Between the Deinococcus-Thermus Group and Cyanobacteria. <i>Journal of Molecular Evolution.</i> Volume 46, Number 6. | |||
( | [4.28] Horneck G. (2000). The microbial world and the case for Mars. <i>Planetary and Space Science.</i> 48 (11), pp. 1053-1063. | ||
[4.29] Stibal, M., Tranter, M., Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. <i>Journal of Geophysical Research</i>, Vol. 112, G04S33, Pg 1-9, Copyright 2007, American Geophysical Union. | |||
===Sea Ice=== | |||
[5.1] | [5.1] Nichols, Carol Mancuso, John P. Bowman, and Jean Guezennec. | ||
“Effects of Incubation Temperature on Growth and Production of Exopolysaccharides by an Antarctic Sea Ice Bacterium Grown in Batch Culture.” Applied Environmental Microbiology. 2005 July; 71(7): 3519–3523. | “Effects of Incubation Temperature on Growth and Production of Exopolysaccharides by an Antarctic Sea Ice Bacterium Grown in Batch Culture.” Applied Environmental Microbiology. 2005 July; 71(7): 3519–3523. | ||
[5.2] | [5.2] United States. Central Intelligence Agency. The World Factbook. | ||
https://www.cia.gov/library/publications/the-world-factbook/print/ay.html | https://www.cia.gov/library/publications/the-world-factbook/print/ay.html | ||
[5.3] | [5.3] Peck, Lloyd S. “Prospects for surviving climate change in Antarctic aquatic species.” | ||
Front Zool. (2005); 2:9. | Front Zool. (2005); 2:9. | ||
[5.4] | [5.4] Morgan-Kiss, Rachael M., et al. “Adaptation and Acclimation of Photosynthetic | ||
Microorganisms to Permanently Cold Environments.” MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Mar. 2006, p. 222–252. | Microorganisms to Permanently Cold Environments.” MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Mar. 2006, p. 222–252. | ||
[5.5] | [5.5] “Antarctic Weather.” www.antarcticconnection.com | ||
[5.6] | [5.6] Gosink, J. J., Staley J. T. “Biodiversity of gas vacuolate bacteria from Antarctic sea | ||
ice and water.” Applied and Environmental Microbiology. 1995 September; 61(9): 3486–3489. | ice and water.” Applied and Environmental Microbiology. 1995 September; 61(9): 3486–3489. | ||
[5.7] | [5.7] BOWMAN, JOHN P., et al. “Diversity and Association of Psychrophilic Bacteria in | ||
Antarctic Sea Ice.” Applied and Environmental Microbiology. Aug. 1997, p. 3068–3078 Vol. 63, No. 8. | Antarctic Sea Ice.” Applied and Environmental Microbiology. Aug. 1997, p. 3068–3078 Vol. 63, No. 8. | ||
[5.8] | [5.8] Biuw, M., et al. “Variations in behavior and condition of a Southern Ocean top | ||
predator in relation to in situ oceanographic conditions.” Proceedings of the National Academy of Sciences. 2007 August 21; 104(34): 13705–13710. | predator in relation to in situ oceanographic conditions.” Proceedings of the National Academy of Sciences. 2007 August 21; 104(34): 13705–13710. | ||
[5.9] | [5.9] Ducklow, Hugh W, et al. “Marine pelagic ecosystems: the West Antarctic | ||
Peninsula.” Philosophical Transactions of the Royal Society Biological Sciences. 2007 January 29; 362(1477): 67–94. | Peninsula.” Philosophical Transactions of the Royal Society Biological Sciences. 2007 January 29; 362(1477): 67–94. | ||
[5.10] | [5.10] Duck, Hugh, Craig Carlson, “Walker smith Bacterial growth in experimental | ||
plankton assemblages and seawater cultures from the Phaeocystis antarctica bloom in the Ross Sea, Antarctica.” MICROBIAL ECOLOGY. (1999) Vol. 19: 215-227. | plankton assemblages and seawater cultures from the Phaeocystis antarctica bloom in the Ross Sea, Antarctica.” MICROBIAL ECOLOGY. (1999) Vol. 19: 215-227. | ||
[5.11] | [5.11] D’Amico, Salvino, et al. “Psychrophilic microorganisms: challenges for life.” | ||
European Molecular Biology Organization reports. (2006) VOL 7 | NO 4, 385–389. | European Molecular Biology Organization reports. (2006) VOL 7 | NO 4, 385–389. | ||
[5.12] | [5.12] Price, Buford P., Todd Sowers. “Temperature dependence of metabolic rates for microbial growth, maintenance, and survival.” Proceedings of the National Academy of Sciences. January 22, 20 | ||
===Freshwater Ice=== | |||
[6.1]: Carpenter, E. J., S. Lin, and D. G. Capone. 2000. Bacterial activity in South Pole snow. Appl. Environ. Microbiol. 66:4514-4517. | |||
[6.2]: Vincent WF, Quesada A (1994) Ultraviolet radiation effects on cyanobacteria: implications for Antarctic microbial ecosystems. Antarctic Res Ser 62:111–124 | |||
[6.3]: Campbell JW, Aarup T (1989) Photosynthetically available radiation at high latitudes. Limnology and Oceanography 34:1490–1499 | |||
[6.4]: Tanabe, et al. (2007). Phytoplankton blooms under dim and cold conditions in freshwater lakes of East Antarctica. Polar biology, 31(2), 199-208. | |||
[6.5]: Jones, A. E., and J. D. Shanklin. 1995. Continued decline of total ozone over Halley, Antarctica, since 1985. Nature 376:409-411 | |||
[6.6]: M. R. James. et al. 1996. Biodiversity in extreme aquatic environments: Lakes, ponds and streams of the Ross Sea sector, Antarctica. Biodiversity Conservation. 5: 145 1-1471. | |||
[6.7]: Vincent WF, Rae R, Laurion I, Howard-Williams C, Priscu JC (1998) Transparency of Antarctic ice-covered lakes to solar UV radiation. Limnology and Oceanography 43:618–624 | |||
[6.8]: Howard-Williams, C. (2007). Ecological processes in Antarctic inland waters: interactions between physical processes and the nitrogen cycle. Antarctic science, 19(2), 205-217. | |||
[6.9]: John C. Priscu, Craig F. Wolf, Cristina D. Takacs, Christian H. Fritsen, Johanna Laybourn-Parry, Emily C. Roberts, Birgit Sattler and Berry Lyons. BioScience, Vol. 49, No. 12, McMurdo Dry Valleys (Dec., 1999), pp. 997-1008 | |||
[6.10]: Arnold, B.R., Foreman, C.M., Mikucki J.A., Priscu, J.C. (2006) (http://www.homepage.montana.edu/~lkbonney/IMAGES/Presentations/ASM2006Brianna.pdf) | |||
[ | [6.11]: Cavicchioli, R. 2006. Cold-adapted Archaea. Nat. Rev. Microbiol. 4:331-343. | ||
[ | [6.12]: Tindall, B. J. 2004. Prokaryotic diversity in the Antarctic: the tip of the iceberg. Microb. Ecol. 47:271-283. | ||
[ | [6.13]: Ellis-Evans, J. (1996). Microbial diversity and function in Antarctic freshwater ecosystems. Biodiversity and conservation, 5(11), 1395-1431. | ||
[ | [6.14]: Stingl, U. (2008). Dilution-to-extinction culturing of psychrotolerant planktonic bacteria from permanently ice-covered lakes in the McMurdo dry valleys, antarctica. Microbial ecology, 55(3), 395-405. | ||
[ | [6.15]: Andrassy, I (2007). Nematodes from saline and freshwater lakes of the Vestfold Hills, East Antarctica, including the description of Hypodontolaimus antarcticus sp n. Polar biology, 30(6), 669-678. | ||
[6]: | [6.16]: Bratina, B. (1998). Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Applied and environmental microbiology, 64(10), 3791-3797. | ||
[ | [6.17] Kepner, R. (2000). UV radiation and potential biological effects beneath the perennial ice cover of an antarctic lake. Hydrobiologia, 427(1-3), 155-165. | ||
[ | Edited by [Brenna Riley, Sabrina Koperski, Rebecca Dickerson, Trevor Mickelson,Timbrely Fong, Srdjan Sonjara], students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 18:24, 22 April 2011
Antarctica
Antarctica is a continent found almost entirely south of the Antarctic Circle. The climate is harsh: it is the coldest, driest place on earth, with the highest winds, and highest average elevation. Antarctica’s average temperature ranges from -80°C to -90°C in the winter, and 5°C to 15°C in the summer. Little precipitation falls, making Antarctica the largest desert in the world. On average, the interior of the continent only receives 50mm (2in), and the coast only about 200mm (8in). Unlike normal deserts, the precipitation does not evaporate. Instead it builds up as snow or ice over hundreds and thousands of years to form the thick ice sheets present today. All of Antarctica receives very little direct sunlight because of the tilt of the earth; in the summer months Antarctica has almost continuous daylight, and in the winter almost continuous darkness.
Slush
Description of Niche
Location
Slush is located on the southernmost continent on the globe, Antarctica, which covers the South Pole and in the surrounding hydro-areas like the Ross Sea. Each type of ice reflects the age and different forms as well as thickness of ice at different stages of development. Slush refers to recently formed ice and is characterized by snow, which is saturated and mixed with water on land or ice surfaces that grow to no more than 10cm thick. [1.1]
Physical Conditions
Slush conditions are constantly changing depending on the salinity of the water and the outside temperature. In freshwater, sea slush is more solid and clear while in more saline condition it is more granular and porous. Slush builds up after a heavy rain or snowfall however other climate conditions can affect the slush in several ways, ultimately changing the slush into different categories of ice. For example, sea slush that is allowed to accumulate and freeze into circular pieces of ice would now be categorized as pancake ice. [1.2]
Affect of Adjacent Communities
Although Antarctica may seem like a harsh environment, many flora and fauna live on the hostile continent mostly around the costal areas. Antarctica is home many different types of flora, which include two species of flowering plants, three hundred and fifty species of mosses and hundreds of species of algae. Also, several species of fauna live here, mostly inhabiting the neighboring marine environment such as emperor penguins and krill. [1.3]
Changing Conditions
Due to the increasing climate change due to human effects such as global warming, Antarctica is sometimes used as a “global warming barometer.” Because of the increasing temperatures, several types of mosses and lichens are able to grow further and further south as increasing periods of thaw occur. [1.3]
Inhabitants
Microbes
Colwellia polaris, discovered by the LExEn (Life in Extreme Environment) group are gram negative, psychrotolerant, aerobic curved rods, 0.6-0.9 x 0.9-4 micrometers. Cell growth occurs from 4-26°C and pH of 5.0-10.00 while optimum growth occurs around 20°C. [1.4]. These microorganisms are able to survive and grow at these extreme temperatures because they have thermally adapted to their environment by adopting a variety of strategies such as cold shock proteins as well as structural modifications that allow membrane fluidity to continue in such cold temperatures. In other words, Colwellia polaris are able to deal with the exponential decrease in chemical reaction rates and the increase in membrane rigidity because their proteins and metabolic enzymes are able to function in such cold environments. See interaction with environment and metabolism.
Other Organisms
Antarctic organisms do not live directly in sea slush however many are present in the surrounding area/continent – see adjacent communities.
Environmental Interaction
Colwellia polaris must be able to stop ice from forming around itself which would render it unable to function in its icy slush environment. In order to combat ice buildup, these organisms produce an extracellular substance called an ice binding protein (IBP) which has a high affinity for ice thus inhibiting it from collecting on the organism itself. Ice inoculated with Colwellia polaris shows irregular ice growth and pitting on the edges of the ice while uninoculated ice shows no signs of these irregularities. Another related defense instrument is their ice recrystalization inhibition (RI) activity which prevents the formation of larger ice crystals. Ice culture mediums of Colwellia polaris and bovine serum albumin (BSA) were flash frozen and allowed to recrystalize. Due to the RI activity the medium exposed to Colwellia polaris showed smaller, less dense ice crystals. These two extracellular mechanisms allow Colwellia polaris to maintain a fluid environment thus prevent freezing injury to the cell membrane. [1.5]
Metabolic Adaptation
One specific example is the thermal adaptation of the 71-kDa protein M1 aminopeptidase which is renamed cold-active aminopeptidase (ColAP) for the cold adapted enzyme. This protein has an optimum pH level of 6-8.5 and temperature of 19°C. ColAP’s main function is to assist in proteolytic cleavage, which is important in bacterial metabolism. Proteolytic activity aids these organisms in acquiring dissolved organic nutrients such as nitrogen-rich organic compounds. [1.6] In general, ColAP is able to maintain “normal” function at cold temperatures due to fewer proline residues, fewer ion pairs and a lower hydrophobic residue content, which contribute to its flexibility, which as mentioned before is essential for survival in cold icy environments. The low number of proline residues affect the backbone structure of the protein, which lead to an increase in local motility of the chain. The fewer number of ion pairs increase flexibility by decreasing the number of stabilizing bonds in the protein and lastly, the lower hydrophobic residues indicate an increase in charged residues which increase the hydrosolubilty of the organism and allow a greater accessibility at the surface of the protein. Furthermore, due to these adaptive abnormalities, ColAP is very sensitive to heat thus exhibiting a decreasing half-life with increasing temperature. For example, at 0°C, the enzyme half-life was measured to be between 18 and 45 hours while at 50°C the half-life was measured to be only 5 minutes. [1.7]
Lake Vostok
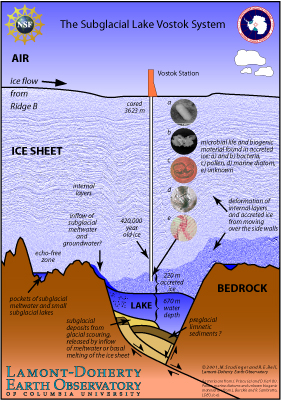
Description of Niche
Lake Vostok lies beneath the Russian Research Station Vostok for which it is named. This subglacial lake has been studied since the late 1960’s yet no one has actually sampled the water within it directly. It is the Largest of the approximately 150+ subglacial lakes [2.1] comparable in size to Lake Ontario with approximately 5000 km3 of water.[2.2, 2.3] It is thought that Lake Vostok has been continuously isolated from the earth’s atmosphere for the past 15-30 million years, also rendering it cut off from new carbon sources as well as light[2.3] making it a very unique environment. The glacial ice moves across the lake at a speed of ~3m per year in an easterly direction.[2.2]
Location
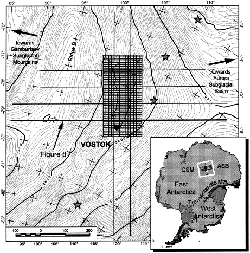
Lake Vostok is located in East Antarctica under approximately 4km of ice. The ice sheath varies in thickness from the northern to the southern region.[2.2]
Physical Conditions
Lake Vostok, like many other subglacial lakes holds many extreme physical conditions, ranging from high pressure ~350 atm, low temperatures (between -3°C and 8°C), and permanent darkness. [2.4] The high pressure exuded from the ice above Lake Vostok keeps is what keeps the lake water in a liquid state, since water freezes at a lower temperature when under high pressure.[2.5]
Affect of Adjacent Communities
Lake Vostok was thought to have been not only isolated from the 20th century atmosphere but also from any other surrounding environments. This thinking has now changed as recent imaging studies have shown that these subglacial lakes are potentially connected through a network of rivers. This makes sterile drilling all the more important, since contamination of one subglacial lake increases the chances of other adjacent subglacial lakes becoming contaminated.[2.2] Also, the actual lake itself is in constant balance with the accretion ice above it. The ice melts in the northern part of Lake Vostok possibly delivering nutrients and other microorganisms into the lake.[2.2]
As the ice in the northern region of Lake Vostok melts it sinks to the lake floor, where it is warmed via geothermal activity and/or pressure. As the water temperature increases, causing the lake water to become less dense it rise to the surface in the south. Once the water has resurfaced in the south it freezes and becomes part of the accreted ice. This water movement is important in considering the ice core samples. As the water rises to the surface it can bring some of the sediment and the organisms which reside on the lake floor or event throughout the varying depths with it causing it to become trapped in the ice. The coring at Lake Vostok has penetrated into the accretion zone in the southern portion of the lake. (The accretion zone is the lowest 210m, and the core sample was taken from 150m above the lake)[2.4]
As the ice moves across any sediment a small amount of the it becomes entrapped.[2.6] According to Christner et al., this provides a mechanism by which organism can get transferred into Lake Vostok.
Changing Conditions
Dependent on the depth of the water the physical conditions of Lake Vostok can vary. Lake Vostok has been previously thought to be an area without any seismic activity – this has now changed. A low amount of seismic activity has been noted. The seismic activity can release organisms into the lake as well as provide a source of heat and nutrition. Furthermore, microseismic activity has been recorded nearby the Vostok station which cold drive convection movement within the Lake.[2.2]
Inhabitants
Currently there is no concrete knowledge on the type of organisms which reside in Lake Vostok. There are many stipulations on the types of organisms, if any, that might be found within any of the subglacial lakes including Lake Vostok. The organisms speculated to inhabit this niche are most likely extremophiles, tolerant of extremely low temperatures (<0°C). It is further postulated that these organisms would probably have an adaptation to high pressure. In recent years continued coring has indeed brought evidence of such microbes. According to Karl, et al. the organisms found within one of the core samples were found to be oligotrophic. Oligotrophic organisms live with minimal nutrients, low biomass, and also a low energy flux,[2.5] which would be consistent with the conditions wihting the lake.
In one experiment core ice from section 3593 was melted and samples of the melt ice were spread on agar plates, enriched with various nutrients and incubated at 25°C. Four colonies were grown and 16S rDNA comparison was performed. This analysis showed that the microbes, which had been cultured, have similar 16S sequences. Their nearest phylogenetic neighbors are Brachybacterium conglomeratum (found in cheese), Sphingomonas sp. (found in Guliya ice core), Paenibacillus amylolyticus (found in soil), Methylobacterium sp. (found as biofilm on cooling fan), and one unidentified organism.[2.6]
Another study found fourteen different isolates which had been cultured on agar enriched with different nutrients. Among these fourteen separate colonies Cruptococcus sp. and Rhodoturula sp.(both in the yeast family) were identified as well as Subtercola sp., sphingomaonas sp. leaving nine remaining to be identified.[2.7]
Microbes
Evidence from past research suggests the presence of thermophilic chemoautotrophic microorganisms in Lake Vostok. [2.3] As of yet we can still only stipulate what types of organisms can be found within the actual Lake as all of the coring samples are from accretion ice. Also, there is a large chance for organisms found within the ice cores to be contaminant, even after the large amount of precautions taken to minimize the risk thereof. So far Researchers have isolated a few novel species which might be found within Lake Vostok:
- Hydrogenophilus gen. nov. is a rod shaped, non sporulating, Gram-negative bacterium, which is a chemolithoautotrophe. These bacteria utilize H+ as their electron donor, with CO2 as their electron acceptor.[2.8]
- Hydrogenophilus thermoluteolus sp. nov. is a heterotrophic bacterium, which grows best at temperatures between 50-52°C and a pH of 7. When grown in colonies it has a dull yellow color. It uses acetate, propionate butyrate, succinate, DL-lactate, pyruvate and α-ketoglutarate as both electron donors and as their carbon source. It uses Ammonium ions, nitrate ions and urea as its nitrogen sources. [2.8]
Environmental Interaction
It has been thought that some of the organisms which reside in such extreme cold have an ability which allows them to change the physical structure of the ice around them. Also, these conditions warrant a slow metabolism as well as an alternate energy source. Ice binding proteins, which inhibit ice-recrystallization have been isolated out of core samples at Lake Vostok.[2.7] Raymond et al. discuss the possibility of bacteria and other microbes forming channels through the ice. These channels would be filled with liquid water as the ice-binding proteins would inhibit the recrystallization. This would be a ground where potential communities of microbes could survive. It is also thought that other microbes, which do not carry the gene for the ice-binding proteins could live along side those that posses this unique ability. The interaction and habitat formation is thought to be similar to that of soil bacteria.[2.7]
Ross Dependency
Description of Niche
Location
The Ross Dependency is located in the South-western part of Antarctica. It is a large area that encompasses most of the Ross Ice Shelf as well as much of the Ross Sea.
Physical Conditions
The average temperature of ice in the Ross Ice Shelf is -2.13 to -2.16° C, slightly colder than -2°C, the temperature at which seawater freezes. The Ross Ice Shelf receives 202.5mm of rainfall per year on average. The average temperature of the Ross Sea is -1.9°C, and it is a salt water sea [3.2].
Changing Conditions
The Ross Dependency region receives the most rain between February and June, an average of 22.5mm/month. Between July and January the amount of rainfall severely decreases to about 12.7mm/month. The average temperature, between -2°C and -10°C, is highest between November and February. From March to October, the average temperature is between -18°C and -26°C [3.5]. The Ross Dependency and all of Antarctica experiences 6 months of continuous sunlight and 6 months of continuous darkness each year.
Inhabitants
Microbes
Many types of phytoplankton live in the Ross Dependency area. These include several species of diatoms, haptophytes, dinoflagellates, and cryptophytes [3.4]. Some of the diatoms that live there are Corethron coriophyllum, Pseudonitschia, Fragilariopsis, Rhizosolenia, and Thalassiosira. Diatoms like Fragilariopsis and Pseudoitschia are found in extensive blooms near ice edges in the summer months [3.4]. Cryptophytes can also be found in large blooms in the Ross Sea. One of the most studied haptophyte that lives in the Ross Dependency is Phaeocystis antarctica. P. antarctica generally live in hollow colonies though solitary cells have been found, as well. Living in colonies helps this bacterium evade predators as single cells are more likely to be ingested. This microorganism, as well as many other bacteria that live in the Ross Dependency, is most prevalent in spring and populations decline drastically in the summer months [3.1]. The tidal zone of the Ross Ice Shelf has a biofilm made of diatoms and cyanobacteria.
Other Organisms
There are no land based vertebrate animals naturally present in the Ross Dependency, or even in Antarctica. Small invertebrate animals inhabit the island. No trees or bushes can survive there, however around 350 species of mosses, lichens and algae live around Antarctica. In addition to the microbes, many types of marine life including larger plankton, krill, fish, seal, penguins, and whales live in the Ross Sea. Phytoplankton are a direct energy source for some of these organisms. Bacteria is the primary food source for microzooplankton. Ice Krill and plankton eat diatoms, and Antarctic toothfish often consume P. Antarctica [3.2].
Metabolic Adaptations
Scientists have found that Antarctic ice, including the Ross Ice Shelf, contains extensive criss-crossing acidic "veins" that can have pH as low as 0. Microbes who live in these veins are thought to extract their energy from the acid surrounding them [3.7]. These acidophiles must also protect themselves from too much acid. In order to keep their intracellular pH ideal, some microbes maintain a positive surface membrane charge, have a membrane that is impermeable to protons, or synthesize proteins that remove protons from the cell. Some microbes may also form spores if they can't regulate intracellular acidity well enough [3.7]. Unlike an activly metabolizing organism, spores do not need to regulate intracellular pH very tightly.
The extremely low temperatures of the South Pole make it very challenging for microbial life to thrive there. Because of these harsh conditions, microbes that live there often have very low metabolic and division rates; some even shut down all unnecessary energy consumption when the temperatures drop. A microbe may have other responses to the cold, including: an increase in production of chaperones and other stress proteins, express starvation genes, or synthesis of "antifreeze proteins" to protect their cytoplasm from freezing [3.7]. Addtionally, microbes living in the Ross Dependency necessarily have a greater percentage of unsaturated fatty acids in their membranes than do warmer-dwelling microbes in order to retain membrane fluidity [3.7].
Environmental Interactions
P. antarctica produces a lot of dimethylsulfide. This is a volatile substance that is necessary for cloud formation. As dimethylsulfide rises into the atmosphere it transforms into aerosols that attract molecules of water, creating clouds [3.3]. Phytoplankton in the Ross Dependency produce chlorophyll. The concentration of chlorophyll in a region can be used to estimate the phytoplankton population at the time. Diatoms and haptophytes also synthesize carbon biomass. This organic matter sinks to a depth of 500 meters by the natural sinking of phytoplankton.
Soil
Description of Niche
Most Antarctic soil is covered with ice from meters to miles thick; only 2% of soil is exposed. [4.1] Inland deserts constitute most of these bare regions, such as the McMurdo Dry Valleys, which receive little to no precipitation. In the Dry Valleys, the equivalent of only 50 mm of rainfall accumulates every year. Temperatures up to a few degrees above freezing, and as low as -89.2° C have been recorded in the center of the continent. [4.2]
Location
Exposed soil is mostly found in the interior of Antarctica in the Mcmurdo Dry Valleys. The coordinates of this region are: 77"00'S, 162O52'E. [4.3]
Soil is also found in Cryoconites. (See Physical Conditions for more information about Cryoconites.)
Physical Conditions
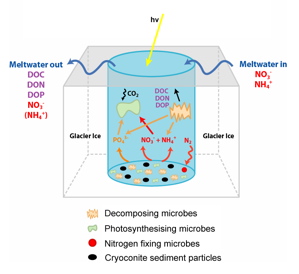
The soil in Antarctica is freezing, arid, low in clay content and organic matter, and high in salinity. It is around 96% sand. There is very little bio-available water. Due to the lack of precipitation, water does not leach cations from the earth, leaving it with an alkaline pH. [4.5] Scientists measured a pH level of 8.51 (+ or - 0.07) in places containing living organisms, though, this is lower than the average soil pH. [4.22]
Cryoconites are water and sediment filled hollows on the surfaces or edges of glaciers. The water and soil mixture periodically freezes and melts. The dark sediment absorbs more light than the white ice and snow, causing it to heat up and melt the surrounding ice. This forms pockets of subsurface liquid water with sediment at the bottom. In the summer, when the covering ice melts, the depressions are open to the atmosphere and receive minerals and microorganisms blown by the wind. The sediment in cryoconites is submerged in water, but is chemically unique from glacial runoff and water found in lakes and rivers. [4.4]
Although not as common as other soil conditions in Antarctica, geothermal activity brings dirt temperature up to 42-60° C, and pH down to 4.5-7.5. Steam vents that produce the heat also add moisture to the earth. They can be found on northwest slope of Mount Melbourne. [4.6]
Affect of Adjacent Communities
The Mcmurdo Dry Valleys also hold rivers and lakes, and are bounded by glaciers and snow pack. Microbes in these niches can interact with those in the dirt. [4.3]
Wind plays a key role in the movement and dispersal of organic matter. [4.20] Dust from the bare land in the McMurdo Valleys is lifted and blown by the wind to rivers, lakes, and glaciers with cryoconites. The dust carries microbes and minerals with it. The microbes caught in cryoconites and in snow can be flushed into lakes by glacial melt. Evaporation can also pick up microorganisms in water and precipitate them elsewhere. These changes in environment promote genetic mixing. [4.4]
One research group suggested that cyanobacteria originate in bodies of water and adapt as they move from water, to mud, to dry, desert soil. They reason that Beacon Valley has a very low cyanobacteria population because it has no lakes or ponds, while in Miers Valley, cyanobacteria florish because bodies of water are present there. [4.7]
Changing Conditions
The Antarctic soil environment changes seasonally. In the McMurdo Dry Valleys during winter, Antarctica is almost continuously dark, the wind can reach speeds of 110 km/h, and the air temperature drops to -60° C. In the summer there is nearly constant daylight, and the temperature oscillates between -35° C and 3° C, depending on cloud cover and wind chill. Most precipitation falls in the summer. When in prolonged direct sunlight, soil and rocks can heat up to 10° C above ambient temperature. [4.8] Lots of sunlight also brings increased solar radiation, which can damage DNA. The problem is exascerbated by the large hole in the ozone layer over Antarctica, which admits even more UV rays. [4.9]
The environment also varies from place to place. In one study looking at the correlation between the living soil communities and abiotic soil composition, soil chemistry and elevation data were collected. The information showed that the amount of nitrogen found in soil samples decreased as elevation increased. This implies that fewer organisms live at higher altitudes, which are colder and tend to have higher salinity. Overall, populations have been described as "patchy," with highly populated regions right next to areas with no life at all because of the varied sediment nutrient content. [4.8]
Scientists are now noticing some effects of oil and other hydrocarbon pollution on the microbe communities. The waste has proved beneficial for some bacterial, and detrimental for others. The overall diversity decreases when hydrocarbon contamination is present. [4.26]
Inhabitants
Microbes
Colonies of bacteria have been found growing in the pores of sandstone rock to avoid exposure to the rapidly changing environment. Organisms that live inside rocks this way are called cryptoendoliths. Endolithic communities are thought to comprise the main forms of life in the polar deserts.
In one study, researchers isolated primarily Cyanobacteria, extremophilic bacteria. and microbial lichen, all primary producing, endolithic organisms, in the soil and rocks of the McMurdo Dry Valleys. To catagorize each individual microbe, they examined their small-subunit (SSU) rRNA genes and matched them with the genes of known microorganisms. They discovered three specific bacteria that were most abundant in their samples. The first, comprising 30% of the sample, is most closely related to the Plectonema genus, (97% rRNA sequence identity). The second, 31% of the sample, is similar to Blastomonas ursincola, (94% rRNA sequence identity). It is representative of α-Proteobacteria, a phylum of aerobic, anoxygenic, phototrophs. The third, making up 26% of the sample, is a microbe with no very close relatives, but with a distant relative from the Deinococcus genus, (90% nucleotide sequence identity). It can be categorized in its own clade called Deinococcus-Thermus. [4.10] Bacteria of the Deinococcus-Thermus phylum are incredibly resistant: they can withstand radiation and extreme temperatures; survive in a vacuum, through droughts and famines; and consume nuclear waste. This phylum is also closely related to Cyanobacteria. [4.27]
They found the remaining percentage to be representatives of green nonsulfur bacteria, Cytophagales, Acidobacteria, and Actinobacteria (6% of clones). Acidobacteria are a phylum of acidophilic bacteria. [4.10] Actinobacteria are gram-postitive bacteria that can decompose strong molecules such as chitin and cellulose. Most are aerobic, but a few are anaerobic. Some Actinobacteria can produce exospores. [4.11] Cyanobacteria, another phylum also known as blue-green algae, obtain energy through photosynthesis. They are Gram-negative, lack flagella, and usually have thick cell walls. [4.12]
In the same study, the dry soil was also found to contain organisms that have rRNA representative of green algae, fungi, and chloroplasts. The isolated fungus is closely related to ascomycete fungus Texosporium sancti-jacobi, while the algae is very similar to Trebouxia jamesii. Each of these species has been identified as a kind of fungi and algae that participates in lichen symbiosis. Because these specific species made up over 70% of the clones produced through PCR for this experiment, they are thought to make up the dominant lichen in the community. Those performing this study mentioned that while they did not uncover all the species in Antarctic soils, they do believe that that have found the main contributors to the energy cycle. [4.10]
In the McMurdo Dry Valleys, Cryoconites support cyanobacteria, photosynthetic algae, heterotrophic bacteria, tardigrades, rotifers, and other microorganisms. [4.4]
A few novel species of microbes have been isolated and identified around steam vents in Antarctica. The first of these is Brevibacillus levickii. Another is most closely related to Alicyclobacillus pomorum (91% similarity). The genus Alicyclobacillus is a group of Gram-positive, heterotrophic, bacteria that form endospores. Both species are thermophilic, and, thus, capable of living at very high temperatures. [4.6]
Populations of Archaea are small and belong to the Group II low-temperature Crenarchaeotes. [4.13]
Early research suggested that diversity was extremely low in the desert inland due to severe environmental conditions, but new experimental methods have shown that the microbial biomass in Antarctic soil is between three to four magnitudes higher than previous estimates, and may have considerably higher diversity, though less diversity than that of milder climates. [4.13]
Specific rRNA sequences, or related species, are only found in very small sections of earth, and not found anywhere else. This implies a large degree of isolation from one community to the next. It also supports the idea that biomass is found in patches surrounded by dead areas. [4.24]
Other Organisms
Certain species of nematodes, springtails, and mites also make the soil and rocks of the Antarctic their home. Those mosses and arthropods may only be able to survive inland in the summer if soil moisture is high enough. [4.13] Nematodes feed on bacteria, but also provide nutrients for bacteria when they die. [4.15] Other invertebrates live in the coastal regions, but are not able to survive in the central portion of Antarctica.
Microbe Interaction
Population density is not high in the desert communities, but since nutrients are so scarce, some competition does occur between different bacterial species. Yet, abiotic factors, such a temperature and soil moisture, are more likely to determine community structure than interaction between organisms (competition, herbivory, predation). [4.13] In fact, biodiversity and population density appears to be most dependent on the amount of moisture in the earth. [4.21]
As noted above, some species of lichens inhabit Antarctica. Lichens are a mutually beneficial arrangement of fungi and single-celled algae called a symbiosis. Together they form a larger, multicellular structure. Fungi cling to rocks to form a foundation on which to live, and compete with other fungi for space. The fungi also give protection and supply water and minerals, while the algae provide nutrients made by photosynthesis. [4.14]
The heterotrophic bacteria eat other microbes, including cyanobacteria. [4.16]
Metabolic Adaptations
A few microorganisms, including bacteria, protozoa, lichens and algae, have stress-resistant, or dormant phases to help them endure subzero temperatures and an intermittent water supply, but these strategies can slow, or halt, metabolism and reproduction. In dormant phase, bacteria can alter their DNA to make it more resistant to UV damage. This is especially important because the hole in the ozone layer over Antarctica admits large amounts of solar radiation. [4.13]
Scientists have analyzed enzymes from psychrophilic (cold-loving) bacteria and compared them to functionally equivalent enzymes from microbes that live in warmer locations. A few modified proteins include: alcohol dehydrogenase, xylanase, α-amylase, β-lactamase, aspartate, citrate synthase, transcarbamylase, subtilisin, Ca2+–Zn2+ protease, malate dehydrogenase and triose phosphate isomerase. These enzymes have higher activity in the cold (0-30°C) than their mesophilic counterparts, but at warmer temperatures the cold enzymes denature. Cold-adapted enzymes have a more flexible structure than those from thermophiles, or mesophiles. This flexibility is probably due to weaker bonds within subunits, fewer ion interactions, weaker intramolecular bonds, stronger solvent interactions, higher amounts of glycyl residues, and lower numbers of prolyl and arginyl residues. The extreme cold makes normal enzyme activity impossible because with normal-strength bonds the structures become too rigid to function as catalysts. The weaker bonds allow the microbe to overcome this problem. [4.17]
Increasing membrane fluidity is another method microbes have developed to adapt to the cold. They have done this by increasing the proportion of unsaturated and polyunsaturated fatty acids. Unsaturated fatty acids have double-bond; double-bonds give fatty acids a lower melting point and viscosity than fatty acids with all single bonds. [4.25]
In periods of frigid cold, most psychrophilic bacteria are in stationary phase (survival phase), rather than growth phase. Photosynthetic activity in Antarctic lichens has been observed at temperatures as low as -17° C. [4.18]
Environmental Interaction
Some cyanobacteria fix nitrogen, though, it is not yet known whether the species found in Antarctica do this. [4.12]
Little is known about endolithic microbial communities because not much research has been performed. They are thought to take part in the weathering of rocks and the cycling of elements and nutrients. [4.10]
Lichen attach to the surfaces of rocks and extract minerals from them. [4.14]
Though oil spills have proven to lower diversity, some species of bacteria can degrade hydrocarbons, thus removing the waste. Some of those that do belong to the phylums Pseudomonas, Sphingomonas, and Rhodococcus. [4.26]
Sea Ice
Description of Niche
Location
Antarctic sea ice surrounds the southernmost continent year around. Due to the 3.5% salinity of the ocean water, sea ice forms at a lower temperature than freshwater: -1.8 °C (28.8 °F) compared to 0 °C (32 °F) [5.1]. The amount of sea ice is consantly changing the size of Antarctica throughout the various seasons. The total land mass of Antarctica without ice is 280,000 km² (108,108.6 sq mi). However, at the peak of winter, Antarctica, when combined with sea ice, reaches a total area of 13,720,000 km² (5,297,321.6 sq mi), which is nearly 1.5 times the size of the United States [5.2]. Sea ice is often confused with icebergs; the other major form of ice surrounding Antarctica. Icebergs are made from either broken-off portions of glaciers or ice shelves. In comparison, icebergs are produced from precipitation and are therefore freshwater, while sea ice is produced from freezing seawater.
Physical Conditions
Temperature
The temperature of the Southern Ocean fluctuates throughout the seasons. In the northerly regions of Antarctica, Signy Island for example, the ocean temperature ranges between -1.8°C in the winter and +1.0°C in the summer [5.3]. However, in more southerly areas, such as McMurdo Sound, the ocean temperature ranges between -2.0°C and -1.7°C annually [5.3]. In comparing the total ocean temperature throughout the Antarctic region, the water temperature rarely fluctuates more than 3°C “making this one of the most thermally stable environments on earth” [5.3]. According to the studies of Lloyd S Peck [5.3], the ocean temperature in the Antarctic region has been low and stable for at least 10 million years.
Light
The amount of light that reaches sea ice varies depending on the time of year. Due to the high latitude, seasonal light “varies between no direct sunlight... to 24 h of direct sunlight” [5.3]. Varying amounts of light causes an enormous shift in sea ice formation. During winter, the light source is limited and decreases the air temperature. This contributes to an overall radiative loss, which causes sea ice to form at a rapid rate. The radiative loss is countered in the summer when the radiative value equals or surpasses that of tropical regions. These opposing radiative values contribute to the annual growth and decline of 10-15 million km2 of sea ice [5.3]. During the summer, phototropic microorganisms that are able to survive atop of the sea ice are exposed to harsh temperatures, high amounts of light, and an increasing amount of UV radiation. Phototropic microorganisms under the sea ice have a more stable environment, yet only have access to less than 1% of light. In contrast, when the Antarctic is between solar light cycles during the winter months, phototropic microorganisms must utilize their evolutionary traits to survive nearly 5 months without light [5.4].
Weather
Antarctica is the coldest and windiest place on Earth. The climate that surrounds Antarctica’s sea ice is some of the most unforgiving on Earth, which is only rivaled by Antarctica’s mountainous terrain. In the winter, the lowest recorded temperature is -89.2°C (-128.6°F) and the highest recorded temperature during summer is +15°C (+59°F). On average the summer temperature is -27.5°C (-17.5°F), contrary to -60°C (-76°F) during the winter. Wind also affects the extreme temperatures of Antarctica. The average inland wind speed is 12 mph. However, on the coast where sea ice is formed, the average wind speed is 198 mph. These high winds in combination with low temperatures make Antarctica the driest desert in the world. These desert conditions arise due to the average humidity revolving around 0.03%, which causes the average precipitation to be less than 1” annually [5.5].
Changing Conditions
Observations of Antarctic conditions have only been recorded for the last 150 years. However, due to limited resources and technology, constant climate monitoring was not possible until the 1950’s [5.5]. Currently, many bases are located on the continent and are equipped with advanced weather recording devices to observe conditions year-round. In addition, satellite technology has been utilized to survey the environment and weather patterns. Above the Antarctic sea ice conditions are constantly changing. Frequent changes in weather alter the landscape of the sea ice consistently. Throughout the year, with the combination of unpredictable temperature changes and hurricane force winds, temporary sea ice fluctuates by 10-15 million km2 [5.3]. However, as previously stated, the ocean’s temperature rarely alters more then 3°C and has done so for more than 10 million years [5.3].
Inhabitants
Microbes
The Southern Ocean has a very low temperature and a high concentration of salt, yet microbial life is able to thrive in these extreme conditions. Microbes that are able to live within the freezing temperatures are known as psychrophilies. Some microbes that live within the sea ice are also halophilic because they are able to live in extremely salty conditions.
According to Nichols [5.1]:
“The sea ice produces highly variable microenvironments in terms of temperature, salinity, nutrient concentration, and light intensities within the columns, which may be as thick as 2 m. Salinity in sea ice brine can range from near that of freshwater to >15% at the ice-seawater interface. Temperatures can range from 0°C to -35°C. Sea ice is thus one of the coldest habitats on earth for marine life.”
Some psychrophilic bacteria have evolved to contain gas vacuoles; this allows them buoyancy to float at a certain level in order to regulate their position in vertically stratified water columns and adapt to their environment [5.6]. These gas vacuole-containing bacteria fall into four main classes: alpha, beta, and gamma Proteobacteria and the Flavobacteria-Cytophaga group. The gas vacuoles give sea ice bacteria an energy-maximizing advantage due to the generally higher enrichment in sea ice compared to other bacteria in open and underlying seawater, which allows bacterial cell biovolumes to grow 5 to 10 times larger [5.7].
Other Organisms
Phytoplankton living in the Southern Ocean are responsible for it being one of the most productive oceans in the world [5.8]. Phytoplanktons are photosynthetic organisms. During the transition from winter to spring, the recession of Antarctic sea ice promotes massive phytoplankton blooms. Due to their sudden growth, the normal chlorophyll level rises from less than 5 μg chlorophyll a/liter, to an amazing 1,000 μg chlorophyll a/liter, which not only helps produce half of the worlds oxygen supply but also perpetuates one of the largest links in the Southern Ocean’s food chain [5.4].
Microbe Interaction
Phytoplanktons produce much of the atmosphere’s oxygen by consuming CO2. Two of the essential nutrients of phytoplankton are nitrate and phosphate. During occasionally large phytoplankton blooms, or in areas of large phytoplankton accumulation, surface nitrate and phosphate may be nearly depleted [5.9]. When the resources are depleted the phytoplankton die off, creating an accumulation of slow-to-degrade dissolved organic matter (DOM), which fuels bacteria production through the winter [5.10]. Utilization of DOM by bacteria creates the byproducts nitrogen and phosphate, which contributes to the renutrification of the ocean.
Metabolic Adaptation
Both bacteria and phytoplankton are psychrophilic microbes. Due to the low temperature, these organisms have adapted and evolved. In order to grow and function, they did as follows: “reduced enzyme activity; decreased membrane fluidity; altered transport of nutrients and waste products; decreased rates of transcription, translation and cell division; protein cold-denaturation; inappropriate protein folding; and intracellular ice formation” [5.11].
Freshwater Ice
Location
The Antarctic is one of Earth's extreme places. Many different species of microbes and flora are unique to Antarctica, suggesting many years of evolutionary isolation. Due to the extreme conditions faced by the multitude of microorganisms inhabiting the continent, many have evolved remarkable biochemical, physiological and behavioral adaptations, the study of which is leading to the discovery of useful chemicals and genes. These organisms live in an environment that is rapidly changing due to the effects of global warming. Antarctica is a unique natural laboratory for investigating the effects of environmental changes on the structure and function of biological communities and their genetic makeup.
Physical Conditions
Approximately 61 percent of all fresh water on the Earth is held in the Antarctic ice sheet, an amount equivalent to 70 m of water in the world's oceans. In East Antarctica, the ice sheet rests on a major landmass, but in West Antarctica the bed can extend to more than 2,500 m below sea level. The land would be seabed if the ice sheet were not there.
Temperatures in the Antarctic can be intense and fluctuate from about -85°C in austral winter, while in summer the temperature can warm to about -13°C (mean monthly air temperature in December is -26°C). Microbes, in particular bacteria, have been cultured from samples taken from Antarctic ice cores, and deep cores from the accreted ice above subglacial Lake Vostok and have revealed a high diversity of species that were reported to be metabolically active when warmed to 3°C [6.1].
Affect of Adjacent Communities
Ancient Antarctic microbial communities have persisted for many years, and throughout their course some have evolved traits to help them survive in complete isolation. Some microbial communities such as those that have evolved to sustain themselves on their own are not affected by adjacent communities, while organisms such as phytoplankton rely on the collective process cycling compounds such as nitrogen and gathering light and nutrients to sustain an existence from the environment.
Changing Conditions
Limnological parameters including: water temperature, light availability, turbidity, and chlorophyll a concentration vary with the seasons. Water is in a liquid phase throughout the year, with temperatures ranging from 0 to 10°C [6.2]
Even though the Antarctic benefits from a continuous amount of sunlight during the summer months, the long and dark winter months result in the lowest annual levels of photosynthetically active radiation (PAR) at the surface of the Earth [6.3]. Not only are polar lakes greatly restricted from sunlight during the winter months, but the presence of ice and snow that cover them further make it difficult for light to reach the photosynthesizing organisms in the water. Antarctic lake environments are considered as extremely low productive ecosystems because of their oligotrophic situation and the low temperatures to which they are constantly exposed. Therefore, the theory is that the growth stage of phototrophs in continental Antarctic lakes (phytoplankton and benthic algae are the main primary producers in these lakes) can take place only during the summer [6.4].
The impact of increasing solar ultraviolet-B radiation (UVB, 280-320 nm) on aquatic ecosystems has been of greatest concern in the southern polar region where the annual depletion of stratospheric ozone now extends from spring into late summer [6.5]. There have been quite a few studies done in regards to the penetration and potential effects of UVB in the Southern Ocean but not much is known about non-marine ecosystems such as lakes, etc. Antarctic lakes and streams have unique microbial ecosystems and contain a species-poor community structure that is limited by extreme isolation and the harsh continental environment [6.6]. These communities must now contend with the additional stress of increasing short-wave ultraviolet radiation. In many lakes in the temperate zone, the aquatic biota are protected from UVB (280-320 nm) and to a lesser extent UVA (320-400 nm) by the presence of chromophoric dissolved organic matter (CDOM). These materials are composed of aromatic humic and fulvic acids brought in from vegetation and leaf litter in the surrounding catchment [6.7].
Inhabitants
Prokaryotes are abundant and active in polar environments [6.11]. Antarctic lakes are particularly interesting in this respect because they are exclusively microbial ecosystems [6.12]. Freshwater lakes occur through much of Antarctica and are characterized by short food chains dominated by microbes.
Microbes and Microbe Interaction
Comparatively few studies have been made of continental freshwater lakes until recently, with the main emphasis being on the less extreme maritime Antarctic lakes. Information on seasonal and spatial patterns of microbial activity for freshwater lakes demonstrates rapid changes in community composition at certain times of year despite constant low temperatures. Benthic communities of cyanobacteria and bacteria are a feature of most lakes and are involved in a wide range of geochemical cycling [6.13].
Lakes in the McMurdo Dry Valleys of Antarctica are characterized by a permanent ice cover and little or no anthropogenic influence. The sequencing of 16S rRNA genes of randomly selected representative bacterial cultures from fresh surface water of Lake Fryxell and the hypersaline, suboxic bottom water from Lake Bonney revealed that the corresponding isolates belonged to the Alphaproteobacteria, Betaproteobacteria, Bacteroidetes, and Actinobacteria. Phylogenetic analysis of the sequences showed that the vast majority of the isolates were not closely related to previously described species [6.14].
The invertebrate fauna of many Antarctic ice-free areas, even those close to permanent research stations, are poorly known. Nematodes from freshwater and saline, marine-derived lakes of the Vestfold Hills, East Antarctica exist. The freshwater lakes contain the widespread East Antarctic endemic species, Plectus frigophilus. The saline lakes were inhabited by two recently described species, Halomonhystera halophila and Halomonhystera continentalis, and by a new species, Hypodontolaimus antarcticus. The nematode fauna of Antarctica now consists of 54 named species, 22 of which are found in East Antarctica [6.15].
Environmental Interaction
In order for ecosystems to sustain themselves, the organisms that inhabit them must possess attributes that are adequate and diverse enough to provide all of the basic pathways that permit the processes of ecosystems to function. These processes include: nutrient cycling, photosynthesis, the range of catabolic pathways (aerobic and anaerobic), nitrogen fixation, the nitrogen and sulphur cycles and trace element metabolism. The nitrogen cycle is an important microbially mediated metabolic pathway present in Antarctic lakes and other aquatic systems [6.8]. Nitrogen uptake and nitrogen fixation have been characterized in inland Antarctic waters and at similar rates to other ecosystems at lower altitudes. However, the unique features of Antarctic ecosystems stem from the extreme and variable physical conditions under which these processes such as the nitrogen cycle operate [6.8].
Antarctic lakes in the McMurdo Dry Valleys contain a complex food web that is driven both by ongoing organic carbon production and carbon left as a legacy of past events. Production of organic carbon by autotrophic organisms and the heterotrophic transformations that follow initiate biogeochemical reactions that influence diversity and abundance of species in all ecosystems. The timing and magnitude of organic carbon production is particularly critical in desert ecosystems, where extreme temperatures and a lack of liquid water limits the period when biological activity can persist. Another stress faced on the system is the seasonal change of available sunlight. It is now clear that the presence of liquid water in the McMurdo Dry Valleys produces a cascade of tightly coupled events that ultimately leads to the biological production and cycling of organic carbon and related elements [6.9]. The lack of water and the sensitive balance between gains and losses of organic carbon make the McMurdo Dry Valleys ecosystem one of the most delicate indicators of environmental change on the planet. Antarctica’s extreme conditions, in which certain chemical, physical, and biological properties are exaggerated make Antarctic lakes ideal experimental systems that offer potential insights into ecosystem structure and function that are not obvious in less extreme systems [6.9].
Metabolic Adaptation
Analysis of metals in Lake Vanda, a permanently ice-covered, stratified Antarctic lake, suggest the importance of particulate manganese oxides in the scavenging, transport, and release of metals. Lake Vanda is filled with manganese-reducing bacteria and based on phylogenetic analysis of the 16S rRNA gene sequence, many manganese reducers are part of the genus Carnobacterium. Whereas anaerobes use manganese oxides as their terminal electron acceptors in cellular respiration, isolates of Carnobacterium were shown to reduce Mn(IV) by means of a diffusible compound under oxic conditions [6.16]. It was shown that the release of adsorbed trace metals accompanying the solubilization of manganese oxides most likely supply populations of Carnobacterium with a source of nutrients, where the environment is very oligotrophic [6.16]
From studies at Lake Hoare, South Victoria Land, Antarctica, exposure of benthic and planktonic microbes to Ultraviolet radiation (UVR) encountered immediately beneath the ice is unlikely to inhibit microbial metabolism [6.17]. Even though waters of oligotrophic antarctic lakes are very transparent to UVR, the thick, high scattering and optically dense ice covers on many of these lakes offers organisms a degree of protection largely unavailable in temperate and tropical systems [6.17]. With the effects of global warming taking effect, thinning or complete loss of these overlying ice covers is likely to have major consequences for the structure of antarctic lake microbial communities.
Current Research
Primary Research
Ongoing research is constantly being done in order to ascertain how psychophillic bacteria are able to function and survive in the cold harsh Antarctic climate. One such adaptation is the presence of antifreeze proteins (AFPs) which allow microbes to live in subzero temperatures. Furthermore, each bacterium may contain different types of AFPs as this group of proteins is very diverse. Only a handful of bacteria are known to have AFPs which include Pseudomonas putida (Sun et al., 1995; Xu et al., 1998; Kawahara et al., 2001), Micrococcus cryophilus, Rhodococcus erythropolis (Duman & Olsen, 1993), Marinomonas protea (Mills, 1999) and a Moraxella species (Yamashita et al., 2002). Scientists hypothesize that the difference between each AFPs are due to the environments the bacterium resides in which in turn shape the evolution of these proteins. As a result, measuring AFP activity of different organisms using the available techniques may not be sufficient. One study takes a look at the differences in AFPs in different Antarctic environments such as freshwater and seawater which range from average freshwater salinity to 240% salinity and then attempts to develop new assays in order to measure AFP activity in each unique organism. One assay developed, called the HTAP assay measures light refraction in the sample. Samples with no AFP activity will appear to be transparent while samples with AFP activity will appear opaque due to the small dense crystals AFP proteins help create. Another assay, the splat assay which uses crossed polarized microscopy, was used to verify positive AFP activity results from the HTAP assay. This was necessary because color pigmentation from other sources, other than the ice itself, may skew the results of the light based HTAP assay. [1.8]
Raymond et al. discuss the possibility of specific adaptations for survival within ice of microorganisms. The Researchers hypothesized that a particular type of ice-binding protein should be present in microbes found in the samples of the ice cores, as a similar adaptation can be found in bacteria which reside in antifreeze. The protein in question is one that specifically alters the structure of the ice crystals around the organism allowing it to survive. Potential contaminants were removed from the exterior of the ice cores as per protocol. Ice samples were melted and subsequently incubated at 4°C. Following incubation the isolates were grown on different media, either R2 or agar-solidified. Fourteen species were isolated (mentioned above). An ice-pitting assay was preformed to determine whether any of these microbes held the ability in question. Only one bacterium showed activity and caused hexagonal pitting. A series of comparisons was undertaken to determine the bacterium's place within the phylogenetic tree. Though it produced ice-binding proteins (IBP) similar to other bacteria living in icy environments the proteins were distinct enough for the chance of the bacterium to be a contaminant to be very small.[2.7]
Biotech and pharmaceutical companies are becoming increasingly interested in compounds extracted from Antarctic life forms These organisms live in harsh frozen conditions and have developed complex mechanisms to deal with the low temperature, lack of nutrients, and other difficulties of living in such an extreme environment. Companies that develop industrial processes that keep food or medicines fresh while at a low temperature are currently conducting research on unique enzymes found in Antarctic organisms that can aid the process of chilling without killing. As of 2004, 154 patents have been obtained for compounds naturally found in the Antarctic, including one for a glycoprotein found in a bacterium that has been shown to heal wounds and skin and benefit hair and nails [3.6]. The problem with this research is that it is difficult to know how much is purely for scientific purposes and how much is for financial gain. Companies looking for novel compounds to turn into drugs for profit may cause environmental damage or exploit the resources found on the greatly undisturbed continent. Fortunately, many Antarctic researchers have expressed this concern and are trying to put regulations in place to prevent abuse of resources [3.6].
P. Buford Price from the Physics Department, University of California, Berkeley, CA and Todd Sowers from the Department of Geosciences, Pennsylvania State University, University Park, PA. performed studies on the metabolic rates in microbes at very low temperatures. Price and Sowers compared many different substances to determine different metabolic rates of microbial communities. Their findings demonstrated that there is no minimum temperature for metabolism [5.12]. In fact, Price and Sowers show that microbes in ice have similar metabolic rates to other substances at the same temperature. Therefore, the data confirms that liquid water inside ice is available for metabolism [5.12].
There is also rising interest in bacteria that are capable of cleaning up oil spills and other pollution in Antarctica. Only cold-adapted organisms would be able to take part in this bioremediation.[4.23]
Hydrocarbon Contamination and Biodegradation Within the Permanent Ice Cover of Lake Fryxell, Antarctica:
Psychrophilic enzymes are of commercial interest because of their ability to carry out reactions at low temperatures and have potential utility in bioremediation. Another goal is to find organisms that can safely degrade toxic organic contaminants (for example, petroleum) in the cold. Artic environments are particularly sensitive to pollutions because contaminants are slow to degrade in the freezing temperatures.
During the summer of 2003, a helicopter crashed onto the permanent ice cover of Lake Fryxell contaminating the surface with several hundred liters of jet fuel (JP8). Researchers examined the influence of this spill on the microbial community that resides within the ice cover [6.10].
The researchers wanted to test whether there are hydrocarbon degrading organisms present in the natural lake ice community. Their experiments measured the degradation of JP8 jet fuel, as well as fractions of this fuel including naphthalene (aromatic) and nonane (C9 alkane)[6.10]. Studies have shown enhanced degradation of hydrocarbons in Antarctic soils with the addition of N and P, therefore our experimental treatments were run with and without additional N and P.
These experiments demonstarted that the native ice community found in Lake Fryxell is capable of degrading JP8 jet fuel and fractions of the fuel. Respirometry experiments showed that addition of N and P increased the rate of degradation, this may be due to the fact that this environment is limited in these nutrients. A change in community and diversity was observed in both the TTGE analysis and culturing methods suggesting that the hydrocarbon spill changed the community structure of the lake ice [6.10].
Researchers continue to discover microbes deeper and deeper within the soil of the Antarctic Dry Valleys. Since Antarctic conditions below the soil or ice are so close to the subzero, parched, high UV radiation, anaerobic, icy environment of polar Mars, many believe that these findings give hope of uncovering microbial life, or fossilized remains of life, on our neighboring planet.[4.19] Furthermore, studies have uncovered evidence that suggests that the global climate on early Earth and Mars were comparable. About 3.5 billion years ago, Mars is believed to have had large amounts of liquid water and temperate weather. The idea is if the at one time or another the planets had analogous physical conditions, and those conditions favored the emergence of life on Earth, it is very possible that life also appeared on Mars. Now that scientists have found life on Earth that can withstand the current climate of Mars, they retain the hope that if life did evolve, some organisms may still be alive. The scientists have also laid out various approaches for determining the presence of life on Mars: direct methods like culturing, observation of cell structure, measurements of activity and chemical markers; and indirect methods such as searching for fossils, coal, and “bioweathering,” and testing the composition of the atmosphere for oxygen and other gases. [4.28]
Antarctica Popular science
Here are some links to Articles about Antarctica which have been published in popular science magazines. http://news.nationalgeographic.com/news/2007/12/071227-antarctica-wetland.html - “Antarctica may contain “Oasis of Life”” http://news.nationalgeographic.com/news/2007/05/070516-deep-sea.html - “Bizarre New Deep-Sea Creatures Found Off Antarctica”
http://www.caml.aq/microbes/index.html - This is a Census of Marine Aquatic life.
Conclusions
Antarctica is a niche inhabited by many diverse microbes. These single-celled organisms have adapted to the severe weather conditions in a variety of different ways. New species with unique attributes are unearthed very frequently. Some of the physical modifications are an increase in membrane fluidity, and a decrease in enzyme intramolecular bonds to make them more flexible. A few species have developed anti-freeze proteins to cope with the low temperatures, and ice binding proteins to prevent ice crystals from forming. Other bacteria use the acid veins in the ice to extract energy and, in order to survive, have developed positively-charged, or proton impermeable membranes, and proton-carrying enzymes. Water-dwelling bacteria have gas vacuoles that make them buoyant, and allow the suns rays to reach them for photosynthesis. When their surroundings become too challenging, microbes often slow their metabolism, halt reproduction, and can even form dormant spores to wait until conditions improve. Additionally, these organisms interact with their environment and are essential to the ecosystem of the Antarctic. Since many larger animals cannot tolerate the Antarctic cold, the microbial communities are the dominant forms of life. Phytoplankton consume C02 and produce much of our atmosphere's oxygen. Bacteria take part in all the nutrient cycles, especially the carbon and nitrogen cycles. A lot of research is currently being conducted to discover more about Antarctic microbe lifestyles. Many scientists want to utilize the exceptionally resilient Antarctic bacteria to remove environmental pollutants, such as fuel-spills and radioactive waste. Other bacteria have seemingly unrelated properties that may be useful in the medical field. Researchers have found bacteria that are so tough that they believe similar microbes may be living on Mars today. The special microorganisms of the Antarctic niche are promising discoveries in all aspects of science.
References
Slush
[1.1] World Meteroplogical Organization Information Site: http://www.dbcp.noaa.gov/seashelp/HtmlIceGlossary.htm#slush
[1.2] National Geographic Data Center - National Satellite, Data, and Information Service. http://www.ngdc.noaa.gov/mgg/image/2minrelief.html (2006)
[1.3] International Polar Foundation Information Site: http://www.sciencepoles.org/index.php?/home/
[1.4] Zhang D, Yong Y, Xin Y, Hong L, Zhou P, and Zhou Y. Colwellia polaris sp. Nov., a psychrotolerant bacterium isolated from Arctic sea ice. International Journal of Systematic and Evolutionary Microbiology (2008) Vol. 58 p. 1931-1934
[1.5] Raymond J, Fritsen C, Shen K. An Ice-binding Protein from an Antarctic Sea Ice Bacterium. FEMS Microbiology Ecology (2007) Vol. 61(2) p. 214-221
[1.6] Huston A, Haeggstrom J, Feller G. Cold Adaptation of Enzymes: Structural, Kinetic, and Microcalorimetic characterizations of an aminopeptidase from the Arctic psychrophile Colwellia and of Human Leuikotriene A4 Hydrolase. Biochemica et Biophysica Acta (2008) June 13.
[1.7] Huston A, Methe B, Deming J. Purification, Characterization, and Sequencing of an Extracellular Cold-Active Aminopetidase Produced by Marine Psychrophile Colwellia Starin 34H. American Society for Microbiology (2004) Vol. 70(2) p. 3321-3328
[1.8] Gilbert J, Hill P, Dodd C, Laybourn J. Demonstration of Antifreeze Protein Activity in Antarctica Bacteria. Microbiology (2004) Vol. 150 p. 171-180
Lake Vostok
[2.1] Inman M. Microbial ecology. The Dark and Mushy Side of a Frozen Continent. Science2007 Jul 6;317(5834):35-6.
[2.2] Studinger M, Bella RE, Karnera GD, Tikkua AA, Holtb JW, Morseb DL, Richterb TG, Kempfb SD, Petersb ME, Blankenshipb DD, Sweeneyc RE, Rystromc VL. Ice cover, landscape setting, and geological framework of Lake Vostok, East Antarctica. Earth and Planetary Science Letters2003;205:195-210.
[2.3] Lavire C, Normand P, Alekhina I, Bulat S, Prieur D, Birrien J-L, Fournier P, Hänni C, Petit J-R. Presence of Hydrogenophilus thermoluteolus DNA in accretion ice in the subglacial Lake Vostok, Antarctica, assessed using rrs, cbb and hox. Environmental Microbiology2006;8(12):2106-14.
[2.4] Siegert MJ, Ellis-Evans JC, Tranter M, Mayer C, Petit JR, Salamatin A, Priscu JC. Physical, chemical and biological processes in Lake Vostok and other Antarctic subglacial lakes. Nature2001 Dec 6;414(6864):603-9.
[2.5] Karl DM, Bird DF, Bjorkman K, Houlihan T, Shackelford R, Tupas L. Microorganisms in the accreted ice of Lake Vostok, Antarctica. Science1999 Dec 10;286(5447):2144-7.
[2.6] Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN. Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environ Microbiol2001 Sep;3(9):570-7.
[2.7] Raymond JA, Christner BC, Schuster SC. A bacterial ice-binding protein from the Vostok ice core. Extremophiles2008 Jul 12.
[2.8] Hayashi NR, Ishida T, Yokota A, Kodama T, Igarashi Y. Hydrogenophilus thermoluteolus gen. nov., sp. nov., a thermophilic, facultatively chemolithoautotrophic, hydrogen-oxidizing bacterium. International Journal of Systematic Bacteriology1999;49:783-6.
Ross Dependency
[3.1] Dennett, Mark R., Mathot, Slyvie, Caron, David A., Smith, Walker O. Jr. and Lonsdale, Darcy J. “Abundance and distribution of phototrophic and heterotrophic nano- and microplankton in the southern Ross Sea”. Elsevier Science Ltd. 2001. [1]
[3.2] “Diversity of Ross Sea Fish”. Science Learning Hub. 25 February 2008. [2]
[3.3] Norris, Katina Bucher. “Dimethylsulfide Emission: Climate Control by Marine Algae?”. ProQuest. November 2003. [3]
[3.4] Smith, Walker O Jr, Ainley, David G., and Cattaneo-Vietti, Riccardo. “Trophic interactions within the Ross Sea continental shelf ecosystem”. Philosophical Transactions of the Royal Society B: Biological Sciences. 6 December 2006. p. 95-106. [4]
[3.5] Ward, Paul. “Antarctica Climate Data and Climate Graphs McMurdo, Amundsen-Scott (South Pole) and Vostok Stations”. Cool Antarctica. 17 August 2008. [5]
[3.6] Williams, Nigel. “Chill Wind Over Antarctic Biodiversity”. Current Biology. 9 March 2004. Volume 14, Issue 5. p.R169-R170. [6]
[3.7] Price, P. Buford. “Life in Solid Ice”. Cornell University Library. 2 July 2005. p. 1-11. [7]
Soil
[4.1] "Antarctica - The World Factbook". United States Central Intelligence Agency (2007-03-08). Retrieved on 2007-03-14.
[4.2] "Weather in the Antarctic". British Antarctic Survey. Retrieved on 2006-02-09.
[4.3] Lyons, W.B et al. 1998. A Late Holocene desiccation of Lake Hoare and Lake Fryxell, McMurdo Dry Valleys, Antarctica. Antarctic Science. Vol 10 (3): pgs 247-256.
[4.4] Foreman, C., B. Sattler, J. Mikucki, D. Porazinska and J.C. Priscu. 2007. Metabolic Activity and Diversity of Cryoconites in the Taylor Valley, Antarctica. Journal of Geophysical Research - Biogeosciences. Pgs 1-43.
[4.5] Powers, Laura E., Diana W. Freckman, Mengchi Ho and Ross A. Virginia. (1995). McMurdo LTER: Soil properties associated with nematode distribution along an elevational transect in Taylor Valley, Antarctica. Antarctic Journal of the United States. 30 (5): 282-283.
[4.6] Imperio T, Viti C, Marri L. (2008). Alicyclobacillus pohliae sp. nov., a thermophilic, endospore-forming bacterium isolated from geothermal soil of the north-west slope of Mount Melbourne (Antarctica). Int J Syst Evol Microbiol. 58 (Pt 1):221-5.
[4.7] Wood, S. et al. (2008). Sources of edaphic cyanobacterial diversity in the Dry Valleys of Eastern Antarctica. The ISME Journal. Vol 2, pgs 308–320.
[4.8] Ho, Mengchi, Ross A. Virginia, Laura E. Powers, and Diana W. Freckman. (1995). Soil chemistry along a glacial chronosequence on Andrews Ridge, Taylor Valley. Antarctic Journal of the United States. Vol 30 (5): 310-311.
[4.9] Hogg I.D., Craig Cary S., Convey P., Newsham K.K., O'Donnell A.G., Adams B.J., Aislabie J., (...), Wall D.H. (2006) Biotic interactions in Antarctic terrestrial ecosystems: Are they a factor? Soil Biology and Biochemistry. 38 (10), pp. 3035-3040.
[4.10] de la Torre, J. R., B. M. Goebel, E. I. Friedmann, and N. R. Pace. (2003). Microbial diversity of cryptoendolithic communities from the McMurdo dry valleys, Antarctica. Appl. Environ. Microbiol. 69:3858-3867. [PubMed].
[4.11] Stackebrandt E, Rainey FA, and Ward-Rainey NL. (1997). Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol. 47:479-491.
[4.12] Mitsui, A. et al. (1986). Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 323, 720–722.
[4.13] Hogg I.D., Craig Cary S., Convey P., Newsham K.K., O'Donnell A.G., Adams B.J., Aislabie J., (...), Wall D.H. (2006). Biotic interactions in Antarctic terrestrial ecosystems: Are they a factor? Soil Biology and Biochemistry. 38 (10), pp. 3035-3040. (same as (9))
[4.14] Rosmarie Honegger. (1991). FUNCTIONAL ASPECTS OF THE LICHEN SYMBIOSIS. Annu. Rev. Plant Physiol. Plant Mol. Biol. Vol 42. pgs 553-7.
[4.15] Hunt, H. W. (1987). The detrital food web in shortgrass prairie. Biology and Fertility of Soils. Vol 3, pgs 1-2.
[4.16] BOOKRAGS STAFF. "Heterotrophic Bacteria". (2005). August 23 2008. <http://www.bookrags.com/research/heterotrophic-bacteria-wmi/>.
[[4.17] Gerday C., Aittaleb M., Bentahir M., Chessa J.-P., Claverie P., Collins T., D'Amico S., (...). (2000). Feller G. Cold-adapted enzymes: From fundamentals to biotechnology. Trends in Biotechnology. 18 (3), pp. 103-107.
[4.18] Rivkina, E. I. Friedmann, C. P. McKay, and D. A. Gilichinsky, E. M. (2000). Metabolic Activity of Permafrost Bacteria below the Freezing Point. Appl Environ Microbiol. 66(8): 3230–3233.
[4.19] Wynn-Williams D.D., Edwards H.G.M. (2000). Proximal Analysis of Regolith Habitats and Protective Biomolecules in Situ by Laser Raman Spectroscopy: Overview of Terrestrial Antarctic Habitats and Mars Analogs. Icarus. 144 (2), pp. 486-503.
[4.20] Lancaster, N. (2002). Flux of Eolian Sediment in the McMurdo Dry Valleys, Antarctica: A Preliminary Assessment. Arctic, Antarctic, and Alpine Research. Vol. 34, No. 3 (Aug., 2002), pp. 318-323.
[4.21] Fell J.W., Scorzetti G., Connell L., Craig S. (2006). Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with <5% soil moisture. Soil Biology and Biochemistry. 38 (10), pp. 3107-3119.
[4.22] Wall D.H., Virginia R.A. (1999) Controls on soil biodiversity: Insights from extreme environments. Applied Soil Ecology, 13 (2), pp. 137-150.
[4.23] Aislabie, J., McLeod, M., Fraser, R. (1998). Potential for biodegradation of hydrocarbons in soil from the Ross Dependency, Antarctica. Applied Microbiology and Biotechnology. Volume 49, Number 2.
[4.24] STEVENS, M.I. and HOGG, I. D.. 2006. Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a global indicator. Vol 1. Pgs 1-13..
[4.25] D’Amico, Salvino, et al. (2006). Psychrophilic microorganisms: challenges for life. European Molecular Biology Organization reports. VOL 7 | NO 4, 385–389.
[4.26] David J. Saul a , Jackie M. Aislabie b, *, Caroline E. Brown a , Lisa Harris a , Julia M. Foght c. (2006). Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiology Ecology. Volume 53, Issue 1, Pages 141-155.
[4.27] Gupta, R. S., Johari, V. (1998). Signature Sequences in Diverse Proteins Provide Evidence of a Close Evolutionary Relationship Between the Deinococcus-Thermus Group and Cyanobacteria. Journal of Molecular Evolution. Volume 46, Number 6.
[4.28] Horneck G. (2000). The microbial world and the case for Mars. Planetary and Space Science. 48 (11), pp. 1053-1063.
[4.29] Stibal, M., Tranter, M., Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. Journal of Geophysical Research, Vol. 112, G04S33, Pg 1-9, Copyright 2007, American Geophysical Union.
Sea Ice
[5.1] Nichols, Carol Mancuso, John P. Bowman, and Jean Guezennec. “Effects of Incubation Temperature on Growth and Production of Exopolysaccharides by an Antarctic Sea Ice Bacterium Grown in Batch Culture.” Applied Environmental Microbiology. 2005 July; 71(7): 3519–3523.
[5.2] United States. Central Intelligence Agency. The World Factbook. https://www.cia.gov/library/publications/the-world-factbook/print/ay.html
[5.3] Peck, Lloyd S. “Prospects for surviving climate change in Antarctic aquatic species.” Front Zool. (2005); 2:9.
[5.4] Morgan-Kiss, Rachael M., et al. “Adaptation and Acclimation of Photosynthetic Microorganisms to Permanently Cold Environments.” MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Mar. 2006, p. 222–252.
[5.5] “Antarctic Weather.” www.antarcticconnection.com
[5.6] Gosink, J. J., Staley J. T. “Biodiversity of gas vacuolate bacteria from Antarctic sea ice and water.” Applied and Environmental Microbiology. 1995 September; 61(9): 3486–3489.
[5.7] BOWMAN, JOHN P., et al. “Diversity and Association of Psychrophilic Bacteria in Antarctic Sea Ice.” Applied and Environmental Microbiology. Aug. 1997, p. 3068–3078 Vol. 63, No. 8.
[5.8] Biuw, M., et al. “Variations in behavior and condition of a Southern Ocean top predator in relation to in situ oceanographic conditions.” Proceedings of the National Academy of Sciences. 2007 August 21; 104(34): 13705–13710.
[5.9] Ducklow, Hugh W, et al. “Marine pelagic ecosystems: the West Antarctic Peninsula.” Philosophical Transactions of the Royal Society Biological Sciences. 2007 January 29; 362(1477): 67–94.
[5.10] Duck, Hugh, Craig Carlson, “Walker smith Bacterial growth in experimental plankton assemblages and seawater cultures from the Phaeocystis antarctica bloom in the Ross Sea, Antarctica.” MICROBIAL ECOLOGY. (1999) Vol. 19: 215-227.
[5.11] D’Amico, Salvino, et al. “Psychrophilic microorganisms: challenges for life.” European Molecular Biology Organization reports. (2006) VOL 7 | NO 4, 385–389.
[5.12] Price, Buford P., Todd Sowers. “Temperature dependence of metabolic rates for microbial growth, maintenance, and survival.” Proceedings of the National Academy of Sciences. January 22, 20
Freshwater Ice
[6.1]: Carpenter, E. J., S. Lin, and D. G. Capone. 2000. Bacterial activity in South Pole snow. Appl. Environ. Microbiol. 66:4514-4517.
[6.2]: Vincent WF, Quesada A (1994) Ultraviolet radiation effects on cyanobacteria: implications for Antarctic microbial ecosystems. Antarctic Res Ser 62:111–124
[6.3]: Campbell JW, Aarup T (1989) Photosynthetically available radiation at high latitudes. Limnology and Oceanography 34:1490–1499
[6.4]: Tanabe, et al. (2007). Phytoplankton blooms under dim and cold conditions in freshwater lakes of East Antarctica. Polar biology, 31(2), 199-208.
[6.5]: Jones, A. E., and J. D. Shanklin. 1995. Continued decline of total ozone over Halley, Antarctica, since 1985. Nature 376:409-411
[6.6]: M. R. James. et al. 1996. Biodiversity in extreme aquatic environments: Lakes, ponds and streams of the Ross Sea sector, Antarctica. Biodiversity Conservation. 5: 145 1-1471.
[6.7]: Vincent WF, Rae R, Laurion I, Howard-Williams C, Priscu JC (1998) Transparency of Antarctic ice-covered lakes to solar UV radiation. Limnology and Oceanography 43:618–624
[6.8]: Howard-Williams, C. (2007). Ecological processes in Antarctic inland waters: interactions between physical processes and the nitrogen cycle. Antarctic science, 19(2), 205-217.
[6.9]: John C. Priscu, Craig F. Wolf, Cristina D. Takacs, Christian H. Fritsen, Johanna Laybourn-Parry, Emily C. Roberts, Birgit Sattler and Berry Lyons. BioScience, Vol. 49, No. 12, McMurdo Dry Valleys (Dec., 1999), pp. 997-1008
[6.10]: Arnold, B.R., Foreman, C.M., Mikucki J.A., Priscu, J.C. (2006) (http://www.homepage.montana.edu/~lkbonney/IMAGES/Presentations/ASM2006Brianna.pdf)
[6.11]: Cavicchioli, R. 2006. Cold-adapted Archaea. Nat. Rev. Microbiol. 4:331-343.
[6.12]: Tindall, B. J. 2004. Prokaryotic diversity in the Antarctic: the tip of the iceberg. Microb. Ecol. 47:271-283.
[6.13]: Ellis-Evans, J. (1996). Microbial diversity and function in Antarctic freshwater ecosystems. Biodiversity and conservation, 5(11), 1395-1431.
[6.14]: Stingl, U. (2008). Dilution-to-extinction culturing of psychrotolerant planktonic bacteria from permanently ice-covered lakes in the McMurdo dry valleys, antarctica. Microbial ecology, 55(3), 395-405.
[6.15]: Andrassy, I (2007). Nematodes from saline and freshwater lakes of the Vestfold Hills, East Antarctica, including the description of Hypodontolaimus antarcticus sp n. Polar biology, 30(6), 669-678.
[6.16]: Bratina, B. (1998). Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Applied and environmental microbiology, 64(10), 3791-3797.
[6.17] Kepner, R. (2000). UV radiation and potential biological effects beneath the perennial ice cover of an antarctic lake. Hydrobiologia, 427(1-3), 155-165.
Edited by [Brenna Riley, Sabrina Koperski, Rebecca Dickerson, Trevor Mickelson,Timbrely Fong, Srdjan Sonjara], students of Rachel Larsen












