Anthracimycin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
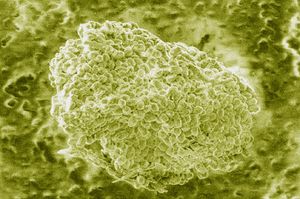
Anthracimycin is an antibiotic discovered in 2012 that is able to kill gram-positive bacteria, namely anthrax (Bacillus Anthracis). It also may be able to kill methicillin-resistant staphylococcus aureus (MRSA), and therefore is an extremely relevant research topic. MRSA is a growing problem in the modern world because of its astonishing capacity to develop drug resistance, so a new antibiotic that defends again MRSA would be a revolutionary discovery. It was found in an ocean microbe called Streptomyces sp. off the shores of Santa Barbara, California. Professor Wiliam Fenical, along with his team of researchers, discovered the compound. | Anthracimycin is an antibiotic discovered in 2012 that is able to kill gram-positive bacteria, namely anthrax (Bacillus Anthracis). It also may be able to kill methicillin-resistant staphylococcus aureus (MRSA), and therefore is an extremely relevant research topic. MRSA is a growing problem in the modern world because of its astonishing capacity to develop drug resistance, so a new antibiotic that defends again MRSA would be a revolutionary discovery. It was found in an ocean microbe called Streptomyces sp. off the shores of Santa Barbara, California. Professor Wiliam Fenical, along with his team of researchers, discovered the compound. | ||
[[image:Image 1241 1-anthrax.jpg|thumb|300px|right| | [[image:Image 1241 1-anthrax.jpg|thumb|300px|right| Electron microscopy view of anthracimycin, from Scinews.com]] | ||
| Line 10: | Line 10: | ||
==Chemical Structure of Anthracimycin== | ==Chemical Structure of Anthracimycin== | ||
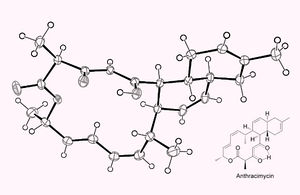
[[image:Image 1241 2-anthrax.jpg|thumb|300px|right| | [[image:Image 1241 2-anthrax.jpg|thumb|300px|right| Chemical structure of anthracimycin, from Scinews.com]] | ||
Anthracimycin, according to Professor Fenical, has a "new and unique chemical structure." New antibiotics do not often have completely unique structures, which is what makes the discovery of anthracimycin particularly important. The chemical formula of anthracimycin is C25H32O4. It is composed of three rings: one has fourteen carbon atoms, and two have six carbon atoms each. These rings are of a polyketide origin, which simply means that the rings have two or more carbonyl groups connected by a single carbon atom. | Anthracimycin, according to Professor Fenical, has a "new and unique chemical structure." New antibiotics do not often have completely unique structures, which is what makes the discovery of anthracimycin particularly important. The chemical formula of anthracimycin is C25H32O4. It is composed of three rings: one has fourteen carbon atoms, and two have six carbon atoms each. These rings are of a polyketide origin, which simply means that the rings have two or more carbonyl groups connected by a single carbon atom. | ||
Revision as of 18:24, 1 December 2013
Introduction
Anthracimycin is an antibiotic discovered in 2012 that is able to kill gram-positive bacteria, namely anthrax (Bacillus Anthracis). It also may be able to kill methicillin-resistant staphylococcus aureus (MRSA), and therefore is an extremely relevant research topic. MRSA is a growing problem in the modern world because of its astonishing capacity to develop drug resistance, so a new antibiotic that defends again MRSA would be a revolutionary discovery. It was found in an ocean microbe called Streptomyces sp. off the shores of Santa Barbara, California. Professor Wiliam Fenical, along with his team of researchers, discovered the compound.
Chemical Structure of Anthracimycin
Anthracimycin, according to Professor Fenical, has a "new and unique chemical structure." New antibiotics do not often have completely unique structures, which is what makes the discovery of anthracimycin particularly important. The chemical formula of anthracimycin is C25H32O4. It is composed of three rings: one has fourteen carbon atoms, and two have six carbon atoms each. These rings are of a polyketide origin, which simply means that the rings have two or more carbonyl groups connected by a single carbon atom.
The technique used to determine the chemical structure of anthracimycin is called spectroscopy.
Method of Action and Potential Uses
The method of action of anthracimycin is not yet determined. Scientists initially hypothesized that it inhibited DNA/RNA synthesis, but follow-up studies (Jang et. al) rejected this hypothesis.
Anthracimycin has been shown to effectively kill gram positive bacteria such as anthrax, which has been used most notably as a bioterrorism weapon. Anthrax is a potentially lethal bacteria, which can require months of treatment with multiple antibiotics. Anthracimycin also showed antimicrobial activity against staphylococci, enterococci, and streptococci. Scientists predict that it may be able to kill MRSA. .
Conclusion
Overall text length should be at least 1,000 words (before counting references), with at least 2 images. Include at least 5 references under Reference section.
References
Aguilera, Mario. "Compound Discovered at Sea Shows Potency against Anthrax." Scripps Institution of Oceanography. 17 July 2013. Web. 05 Nov. 2013.
"Anthracimycin: New Antibiotic Kills Anthrax, MRSA." Scinews.com. 19 July 2013. Web. 05 Nov. 2013.
"Anthrax Killer from the Sea." Chemistry Views. 01 July 2013. Web. 7 Nov. 2013.
Jang, Kyoung H, et. al. "Anthracimycin, a Potential Anthrax Antibiotic from a Marine-Derived Actinomycete." Angewandte Chemie International Edition 52.30 (2013): 7822-824. Wiley Online Library. Web. 7 Nov. 2013.
"Polyketide." Merriam-Webster. Web. 7 Nov. 2013.
Edited by [Emily Vachon], student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2013, Kenyon College.