Antibacterial Surfaces: Difference between revisions
No edit summary |
|||
| (68 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Overview of Antibacterial Surfaces== | ==Overview of Antibacterial Surfaces== | ||
<br> Outside of the body, bacterial control has traditionally been conducted by applying temporary agents like bleach, or through controlled temperature/pressure. The study of antibacterial surfaces investigates the viability of surfaces that intrinsically destroy bacteria and hamper future growth. A number of novel approaches are being employed in the search of effective antibacterial activity. Most of these approaches involve an adhesive coating on benign objects such as glass and polymers. This technology has great potential for medical, commercial, and home use. <br> | <br> Outside of the body, bacterial control has traditionally been conducted by applying temporary agents like bleach, or through controlled temperature/pressure. The study of antibacterial surfaces investigates the viability of surfaces that intrinsically destroy bacteria and hamper future growth. A number of novel approaches are being employed in the search of effective antibacterial activity. Most of these approaches involve an adhesive coating on benign objects such as glass and polymers, or by covalently modifying the surface. Much of the current body of research involves the most efficient ways of preparing the active surfaces. Surfaces destined for use within the oral cavity and within the human body have not been extensively researched in vivo. The overall activity of entire objects (tables, walls, etc.) has also not been tested. A distinction among these various techniques is whether or not the antibacterial agent releases over time. Therefore, certain surfaces have only a limited period of activity before it must be replenished.<br> | ||
<br>This technology has great potential for medical, commercial, and home use. Implanted devices which occasionally cause infection such as pacemakers, catheters, and endotracheal tubes could be rendered bacteria-free. Objects that come in constant human contact, such as door handles, could be treated to prevent bacterial transfer. Entire walls, floors, and ceilings could potentially be affixed with this manner of substance.<br> | |||
==Covalent Modification: Alkylated Polethylenimine== | ==Covalent Modification: Alkylated Polethylenimine== | ||
<br>The covalent attachment of long-chained hydrophobic cations to glass and plastic surfaces can render the modified surface capable of killing bacteria on contact. This method does not involve the release of antiseptics – the long-chained cations intrinsically destroy bacteria upon contact, by virtue of its structure. Unlike other surface treatments, no regeneration of the active material is required, aside from clearing debris from the hydrophobic cations, which can simply be done by washing with detergent. A wide variety of surfaces and textiles can be rendered bactericidal this way. As an added benefit, it appears that bacteria will have little opportunity to adapt against this threat. By directly disrupting the cell wall, this treatment avoids the biochemical route which often leads to resistance. Furthermore these polymers lack any close natural analogs, indicating that resistance development will be very unlikely [7]. <br> | |||
[[Image:PVP.JPG|thumb|300px|left| Fig. 1. "Bactericidal activity of polyethylene slides covalently coated with hexyl-PVP against wild-type and various antibiotic-resistant strains of S. aureus. Either airborne (shaded bars) or waterborne (dashed bars) bacterial suspensions were deposited onto the slide surface; see text for details. All measurements were done in duplicate; the standard deviations from the mean killing efficiency values are represented by error bars." [http://www.biology.neu.edu/pdf/Lin2002.pdf Lin et al., 2002. Insights into bactericidal action of surface-attached poly(vinyl-N-hexylpyridinium) chains.]]] | |||
[[ | <br>In a study by Lin et al., poly(vinyl-N-hexylpyridinium) chains were found “equally lethal against wild-type and mutant, including antibiotic-resistant, strains of the ubiquitous pathogenic bacterium Staphylococcus aureus,” killing between 90-99% of the S. aureus bacteria. [1] This was measured by exposing both airborne and waterborne bacteria to hexy-PVP coated polyethylene slides. The airborne microbes were sprayed onto the surface, while the waterborne microbes were suspended in solution and immersed with the surface with periodic shaking. [1] These hexyl-PVP chains kill a wide variety of both Gram-positive and Gram-negative bacteria, and the activity of the surface can be reset simply by washing it with detergent [1]. A wide variety of multidrug resistant (MDR) bacterial strains were also destroyed by exposure to hexy-PVP. As seen in figure 1, there appears to be little distinction whether the strain was drug-resistant or not - both wild-type and MDR strains were destroyed with exceeding aptitude. This suggests that the mechanism of PVP bactericidal activity is entirely different from that of antibiotics. It is proposed that the chains penetrate the bacterial cell wall/ membrane, and cause lysis by severing the structures at many points. The cationic pyridine nitrogen groups - the repeated unit in each polymer - provides a net positive charge that allow the polymer to puncture the membrane. As polymer length increases, the net charge increases and rigidity is provided both by intramolecular repulsion and repulsion of adjacent cationic polymers (Fig. 2). This novel antibacterial agent has such an overarching, destructive effect on bacteria that it will be very difficult for them to adapt. Unlike the molecular structures of antibiotics, the cationic PVP chains have no biological analog - thus bacteria are not expected to easily adapt a resistance. Only one form of resistance against these molecular “daggers” is known right now, and that is conferred by MDR pumps. The NorA MDR pump of S. aureus protects the cell by expelling amphipathic cations [1]. Mutants with a knockout in the norA gene were compared to their “pump-competent parents,” exhibiting a small difference in killing efficiency of hexyl-PVP: ~99% of airborne/waterborne of pump-lacking mutants were killed, while ~95% of airborne and ~97% of waterborne pump-competent cells were killed [1]. It is believed that the extracellular protrusion of MDR pumps aid in keeping the bacteria at “an arms length” away from the hexyl-PVP antiseptic, rather than specifically binding and expelling hexyl-PVP. It therefore seems unlikely that resistance to surface hexyl-PVP will come to fruition through MDR pumps. Surfaces such as plastic and glass could be made permanently antibacterial after being covalently bound to hexyl-PVP. This presents a wide set of possibilities for limiting bacterial growth in both indoor and outdoor environments. These surfaces seem capable for a variety of uses, especially since it has activity against both waterborne and airborne bacteria (Fig. 1). Water treatment is a possible use for this sort of material, particularly since very little (if any) agent will dissociate into solution. Hypothetically, even floors in high-traffic areas such as hospitals could be given this treatment, radically decreasing the amount of bacteria in the hospital. Furthermore, all manner of intracutaneous devices can be treated this way to prevent post-surgery sepsis – many deaths occur from bacteria embedded on such devices during insertion into the human body.<br> | ||
<br>Previous attempts to design antibacterial surfaces in this manner were largely unsuccessful, because the “polymer chains weren't sufficiently long and flexible to penetrate bacterial cell walls” [ | [[Image:7922notw3.ce.JPG|thumb|300px|right| Fig. 2. Highlights the active portion of hexyl PVP. [http://pubs.acs.org/cen/topstory/7922/7922notw8.html Chemical and Engineering News, Copyright © 2002 American Chemical Society.]]] | ||
<br>Previous attempts to design antibacterial surfaces in this manner were largely unsuccessful, because the “polymer chains weren't sufficiently long and flexible to penetrate bacterial cell walls” [8]. The addition of a linker portion to extend the N-alkylated pyridine groups and a “sweet spot” range of 3-8 PVP chains per unit has fixed this problem (Fig. 2). As seen in figure 2, chains that are either shorter or longer than this range have significantly decreased bactericidal action. Chains that are too short are presumably unable to penetrate the plasma membrane. On the other hand, excessively long chains are overly flexible, and are not rigid enough to be guided through the membrane. The 3-8 unit hydrophobic chains are produced with enough positive charge so that they repel each other while staying flexible, so that they are guided into bacterial cell walls when in close proximity [8]. As mentioned previously, intramolecular repulsion of the cationic groups also provides rigidity to the polymers, although this effect is apparently negated when the polymer is too long.<br> | |||
[[Image:AntiBacPEI.JPG|thumb|350px|left| Fig. 3. "Microbicidal efficiencies of four textiles derivatized with Nalkylated | |||
750-kDa PEI against airborne bacteria and fungi." [http://www.ncbi.nlm.nih.gov/pubmed/12768622 Lin et al., 2002. Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine.]]] | |||
<br>Hydrophobic polycations can also be covalently bound to porous materials such as cotton, wool, nylon, and polyester. Like hexyl-PVP, N-hexylated+methylated highmolecular-weight polyethylenimine (PEI) has strong antibacterial activity against both Gram-positive and Gram-negative bacteria [6]. A variety of microbes have been shown to perish on different kinds of PEI-laden textiles, such as C. albicans, P. aeruginosa, E. coli, and S. aureus (Fig. 3). This was tested by spraying solution-suspended bacteria and fungus/yeast onto the various textile surfaces. The surfaces themselves were prepared by covalently immobilizing PEIs through the following steps: 1. Acylation of hydrolyzed hydroxyl or amino groups of textile with 4-bromobutyryl chloride; 2. Covalent attachment of PEI; and 3. Processing of PEI to produce N-hexyl-PEI. [6] On top of the widespread antibacterial activity, textiles that are covalently bound with N-alkylated PEIs have anti-fungal (airborne) activity. This technique would be particularly useful for producing bactericidal clothes. Hospitals could be rendered bacteria-free by bedsheets and clothing that actively destroy even the most resilient multi-drug resistant bacteria. It could also be rendered a valuable defense against bioterrorism, as surmised by Lin et al. [6]. A possible limitation with this technique is that N-hexyl_PEI may "fall off" of clothing through washing and wear. In order to test continued the continued activity of antibacterial textiles, derivatized cotton was subject to simulated "laundry cycles". After two separate washes that "that included stirring overnight in soapy, warm water, followed by thorough rinsing with distilled water," the antibacterial efficiency of the large (750 kDa) N-hexyl-PEIs were unchanged. [6] However, the smaller PEI chains (0.8 - 2 kDa) showed decreased activity per wash. For this and several other mechanistic reasons, it was determined that the heavier N-hexyl-PEI polycations were of greatest interest for clothing implementation.<br> | |||
[[Image:8023scit1.ce.JPG|thumb|250px|right| Fig. 4. Structure of DABCO, a cationic molecule. [http://www.atsweb.neu.edu/lewislab/publications/cen5.htm Chemical and Engineering News, Copyright © 2002 American Chemical Society.]]] | |||
<br> Alternative forms of alkyl chains with cationic portions are also being researched. A separate research group has devised a method for covalently attaching 1,4-diazabicyclo[2.2.2]octane (DABCO) to five different carbohydrate-based surfaces [8]. Each DABCO molecule is bound at one end to the surface carbohydrate group, and bound at the other end with a lipophilic alkyl chain of variable length (Fig 4.). The DABCO complex with a 16-carbon alkyl chain was found to have the greatest antibacterial activity, destroying both Gram-positive and Gram-negative strains. Like hexyl-PVP and N-alkylated PEIs, DABCO-hexadecane kills on contact and is permanently active [8]. It has also exhibited anti-fungal effects. Since initial testing, the DABCO complex has also been successfully bound to wool and silk. <br> | |||
<br>It has not been determined whether covalently bound long-chained hydrophobic cations disrupt human cells upon contact. This is a potential concern, especially for intracutaneous devices.<br> | <br>It has not been determined whether covalently bound long-chained hydrophobic cations disrupt human cells upon contact. This is a potential concern, especially for intracutaneous devices.<br> | ||
==Silver Nanoparticles== | ==Silver Nanoparticles== | ||
[[Image:Chen ShuiXiaAgACF.JPG|thumb|400px|right|Distribution of silver particles on activated carbon fibers. [http://www. | [[Image:Chen ShuiXiaAgACF.JPG|thumb|400px|right|Figure 5. Distribution of silver particles on activated carbon fibers. [http://www.springerlink.com/content/f7045206n8637v07/ Chen et al., 2005.]]] | ||
<br>Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [5]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria.<br> | <br>Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [5]. Ag+ prevents DNA synthesis by directly binding to DNA [Yoshinari]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria. Ag+ interferes with membrane synthesis by forming Sulfur-Ag bonds, and by adhering to membrane proteins [Yoshinari].<br> | ||
<br>When loaded with Ag through adsoption, Nano-SiO¬2 gains antibacterial properties. According to Wang et al., this antibacterial agent has a “nearly spherical structure with average particle size about 60 nm.” [2] When grafted to wool, Ag-loaded nano-SiO¬2 can destroy up to 84% of E. coli and 94% of S. aureus. [2] This manner of antibacterial surface is limited by the weak bonding between the composite particles and the wool surface. Despite this, “even after washed for 20 times, the grafted wool gave an antibacterial ratio of around 70%,” indicating that this bonding is strong enough to keep a majority of the agent associated with the surface. [2] This type of method is proposed as a way for “fabrics such as medical clothes, protective garments, and hygienic textiles” to become bio-protective [2]. <br> | <br>When loaded with Ag through adsoption, Nano-SiO¬2 gains antibacterial properties. According to Wang et al., this antibacterial agent has a “nearly spherical structure with average particle size about 60 nm.” [2] When grafted to wool, Ag-loaded nano-SiO¬2 can destroy up to 84% of E. coli and 94% of S. aureus. [2] This manner of antibacterial surface is limited by the weak bonding between the composite particles and the wool surface. Despite this, “even after washed for 20 times, the grafted wool gave an antibacterial ratio of around 70%,” indicating that this bonding is strong enough to keep a majority of the agent associated with the surface. [2] This type of method is proposed as a way for “fabrics such as medical clothes, protective garments, and hygienic textiles” to become bio-protective [2]. <br> | ||
<br>Nano-SiO¬2 is a resilient structure that can also carry other inorganic antibacterial elements, such as zinc [4]. Zeolite, phosphates, apatite, and titanium oxides have also been successfully adhered to nano-SiO2. Depending on the amount of metal present, the contact time needed to kill bacteria range from 2 to 10 hours [4].<br> | <br>Nano-SiO¬2 is a resilient structure that can also carry other inorganic antibacterial elements, such as zinc [4]. Zeolite, phosphates, apatite, and titanium oxides have also been successfully adhered to nano-SiO2. Depending on the amount of metal present, the contact time needed to kill bacteria range from 2 to 10 hours [4].<br> | ||
<br>In the treatment of water, activated carbon fiber (ACF) has been used to remove pollutants via adsorption. These fibers encompass a very large surface area, and contain many pores that “collect” pollutants suspended in water. The activity of these fibers can be hampered by the growth of bacteria that adhere to the micropores. The fusing of silver with ACF represents a countermeasure against this bacterial incursion. Chen et al. examined the antibacterial activity of silver supporting activated carbon fibers (ACF-Ag), finding it extremely effective against E. coli and S. aureus [3]. Antibacterial activity was determined to be directly related to the amount of silver adhered to ACF. Interestingly, water treatment was aided by leaving enough of the ACF specific surface area available for bacterial adsorption, bringing said bacteria into closer contact with the silver. After being employed for bacterial destruction, the activity of ACF-Ag was regenerated by washing it with deionized water. After 5 cycles of washing, no reduction in ACF-Ag antibacterial activity was observed [3]. A potential concern with this method of water purification is over-exposure to suspended silver. Under the conditions of Chen et al., whereby 50 mg of ACF-Ag complex was soaked and shaken in 50 ml water for several hours, the maximum amount of silver released was around 10 ppm, meeting “the quality standard of drinking water” [3]. Most water treatment does not use this much ACF per amount of water, so it is surmised that the concentration of released silver will be even lower. Since silver can become permanently deposited in various human body tissues, it is important to keep the concentration of released silver low.<br> | [[Image:AG.JPG|thumb|350px|right|Figure 6. "Antibacterial test against E. coli of Ag-supporting ACF | ||
derived from different precursors". [http://www.springerlink.com/content/f7045206n8637v07/ Chen et al., 2005.]]] | |||
<br>In the treatment of water, activated carbon fiber (ACF) has been used to remove pollutants via adsorption. These fibers encompass a very large surface area, and contain many pores that “collect” pollutants suspended in water. The activity of these fibers can be hampered by the growth of bacteria that adhere to the micropores. The fusing of silver with ACF represents a countermeasure against this bacterial incursion. Chen et al. examined the antibacterial activity of silver supporting activated carbon fibers (ACF-Ag), finding it extremely effective against E. coli and S. aureus [3]. Different silver-supporting activated carbon fibers exhibited antibacterial potency, although sisal based ACF (SACF) was by far the best surface, due to its porousness. (Fig. 5). The viscose and pitch based ACFs were deemed to smooth, and their hydrophobic properties made it more difficult for attached silver to interact with bacteria.[3] Therefore, bacteria cultures were "uc" (uncountable) because little VACF/PACF bactericidal activity occured. Antibacterial activity was determined to be directly related to the amount of silver adhered to ACF. Interestingly, water treatment was aided by leaving enough of the ACF specific surface area available for bacterial adsorption, bringing said bacteria into closer contact with the silver. The theory for this mechanism is supported by the higher activity of the porous SACF, as opposed to VACF or PACF. After being employed for bacterial destruction, the activity of ACF-Ag was regenerated by washing it with deionized water. After 5 cycles of washing, no reduction in ACF-Ag antibacterial activity was observed [3]. A potential concern with this method of water purification is over-exposure to suspended silver. Under the conditions of Chen et al., whereby 50 mg of ACF-Ag complex was soaked and shaken in 50 ml water for several hours, the maximum amount of silver released was around 10 ppm, meeting “the quality standard of drinking water” [3]. Most water treatment does not use this much ACF per amount of water, so it is surmised that the concentration of released silver will be even lower. Since silver can become permanently deposited in various human body tissues, it is important to keep the concentration of released silver low.<br> | |||
<br>Ag-loaded nano-SiO2 also presents a potential health hazard, due to the high rate of dissociation from wool when washed. While silver is a fairly harmless element for people, nano-SiO2 structures could potentially build up in our bodies after constant exposure. | <br>Ag-loaded nano-SiO2 also presents a potential health hazard, due to the high rate of dissociation from wool when washed. While silver is a fairly harmless element for people, nano-SiO2 structures could potentially build up in our bodies after constant exposure. | ||
<br> | <br> | ||
==Other Methods== | ==Other Methods== | ||
<br> | <br>According to Yoshinari et al., the surface of titanium can be modified as to render it antibacterial [9]. This has potential use for providing safer titanium dental implants, particularly for ones that are constantly exposed to the oral cavity. Even though titanium surfaces are highly polished, bacteria are still capable of adhering to these implants. This treatment process including ion implantation, oxidation of the surface, and ion plating on pure titanium plates were found to “significantly inhibit the growth of both P. gingivalis and A. actinomycetemcomitans” [9]. The growth of mouse fibroblast cells was not inhibited in the presence of the modified titanium, indicating that this method is safe for human use. While fluorine ions were implanted into the titanium, no significant release of F+ was detected. The proposed antibacterial mechanism involves a “metal fluoride complex” that forms on the surface of titanium during the dry surface modification [9]. The complex appears to act on bacteria once they’ve adhered to the surface, explaining why the non-adhesive fibroblast cells continued to grow unharmed. Because relatively little F+ is released, this appears to be safe for use in vivo. Fluoride is a widely used antibacterial agent in dentistry, because released fluorine ions have an inhibitory effect on bacterial metabolic enzymes.<br> | ||
<br>A study by Oyane et al. has revealed the use of immobilizing various natural antibacterial agents on the surface of an ethylene-vinyl alcohol copolymer (EVOH) [10]. Percutaneous (within-skin) devices such as catheters are now being designed to achieve maximum adhesion of the device to skin. When coated with a laminin-apatite layer, EVOH exhibits a very close association with skin, however it cannot inhibit bacteria that may be deposited on its surface [10]. The agents lactoferrin, tetracycline, and gatifloxacin were chosen for their effectiveness against E. coli and S. aureus, and were successfully immobilized on the EVOH surface within the laminin-apatite layer. The method of antibacterial activity is based on release of the agent- lactoferrin, tetracycline, and gatifloxacin disassociate from the EVOH surface at varying rates. These antibiotics then proceed to inhibit bacterial growth once released. For further study, the “ideal” agent would have high efficacy against the target bacteria, coupled with a slow release rate [10]. The initial stages of percutaneous device insertion are the most sensitive to bacterial infection, where the skin has not yet formed a tight association with the device. Therefore, permanent anti-bacterial activity is not as important in this circumstance. | |||
.<br> | |||
==Conclusion== | ==Conclusion== | ||
<br> | <br>The subject of antibacterial surfaces encompasses an extremely wide array of ongoing research. Current strategies have yet to be tested in field conditions, so it is difficult to estimate the large-scale bactericidal effect. Three concerns arise in development of these surfaces: cost effectiveness, safety, and microbial adaptation. Given the static nature of many surfaces, it is crucial to design an active surface that microbes have difficulty adapting to.<br> | ||
<br> The above examples are by no means an exhaustive list of the uses of antibacterial surfaces. If these materials are shown to have an acceptably small impact on environmental and human health, it is very likely that their use will become widespread and pervade the world of manufacturing.<br> | |||
==References== | ==References== | ||
[1] [http://www.biology.neu.edu/pdf/Lin2002.pdf Lin, J., Tiller, J., Lee S., Lewis K., and Klibinov, A. “Insights into bactericidal action of surface-attached poly(vinyl-N-hexylpyridinium) chains”. Biotechnology Letters. 2002. Volume 24, Issue 10. p. 801-805. ] | [1] [http://www.biology.neu.edu/pdf/Lin2002.pdf Lin, J., Tiller, J., Lee S., Lewis K., and Klibinov, A. “Insights into bactericidal action of surface-attached poly(vinyl-N-hexylpyridinium) chains”. Biotechnology Letters. 2002. Volume 24, Issue 10. p. 801-805. ] | ||
[2] [http://www. | [2] [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TVV-4NYSX74-7&_user=7774802&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000062877&_version=1&_urlVersion=0&_userid=7774802&md5=1841a6bbe60421a237015f331cf68551 Wang, S., Hou, W., Jia, H., Liu, X., and Xu, B. “Antibacterial activity of nano-SiO2 antibacterial agent grafted on wool surface”. Surface Coatings & Technology. 2007. Volume 202. p. 460-465.] | ||
[3] [http://www.springerlink.com/content/f7045206n8637v07/ Chen, S., Liu, J., and Zeng, H. “Structure and antibacterial activity of silver-supporting activated carbon fibers”. Journal of Materials Science. 2005. Volume 40. p. 6223-6231.] | |||
[4] [ Jia, H., Hou, W., Wei, L., Xu, B., and Liu, X. “The structures and antibacterial properties of nano-SiO2 supported silver/zinc-silver materials”. Dental Materials. 2008. Volume 24. pg. 244-249.] | |||
[5] [ Lok, C., Ho, C., Chen, R., He, Q., Yu, W., Sun, H., Tam, P., Chiu, J., and Che, C. “Silver nanoparticles: partial oxidation and antibacterial activities”. J. Biol. Inorg. Chem. 2007. Volume 12. p. 527-534. ] | |||
[6] [http://www.ncbi.nlm.nih.gov/pubmed/12768622 Lin, J., Qiu, S., Lewis, K., and Klibanov, A. “Mechanism of Bactericidal and Fungicidal Activities of Textiles Covalently Modified With Alkylated Polyethylenimine”. Biotechnology and Bioengineering. 2003. Volume 83, No. 2. p. 168-172.] | |||
[7] [http://pubs.acs.org/cen/topstory/7922/7922notw8.html Borman, S. “DESIGNED SURFACE KILLS BACTERIA”. Chemical & Engineering News. 2001. Volume 79, No. 22. p. 13.] | |||
[8] [http://www.atsweb.neu.edu/lewislab/publications/cen5.htm Borman, S. “Surfaces Designed to Kill Bacteria”. Chemical & Engineering News. 2002. Volume 80, No. 22. p. 36-38.] | |||
[9] [ Yoshinari, M., Oda, Y., Kato, T., and Okuda, K. “Influence of surface modifications to titanium on antibacterial activity in vitro.” Biomaterials. 2001. Volume 22, p. 2043-2048.] | |||
[10] [ Oyane, A., Yokoyama, Y., Uchida, M., and Ito, A. “The formation of an antibacterial agent-apatite composite coating polymer surface using a metastable calcium phosphate solution.” Biomaterials. 2006. Volume 27, p. 3295-3303.] | |||
| Line 52: | Line 80: | ||
Edited by Charles Halsted, student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2009, [http://www.kenyon.edu/index.xml Kenyon College]. | |||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | |||
Latest revision as of 20:12, 10 August 2010
Overview of Antibacterial Surfaces
Outside of the body, bacterial control has traditionally been conducted by applying temporary agents like bleach, or through controlled temperature/pressure. The study of antibacterial surfaces investigates the viability of surfaces that intrinsically destroy bacteria and hamper future growth. A number of novel approaches are being employed in the search of effective antibacterial activity. Most of these approaches involve an adhesive coating on benign objects such as glass and polymers, or by covalently modifying the surface. Much of the current body of research involves the most efficient ways of preparing the active surfaces. Surfaces destined for use within the oral cavity and within the human body have not been extensively researched in vivo. The overall activity of entire objects (tables, walls, etc.) has also not been tested. A distinction among these various techniques is whether or not the antibacterial agent releases over time. Therefore, certain surfaces have only a limited period of activity before it must be replenished.
This technology has great potential for medical, commercial, and home use. Implanted devices which occasionally cause infection such as pacemakers, catheters, and endotracheal tubes could be rendered bacteria-free. Objects that come in constant human contact, such as door handles, could be treated to prevent bacterial transfer. Entire walls, floors, and ceilings could potentially be affixed with this manner of substance.
Covalent Modification: Alkylated Polethylenimine
The covalent attachment of long-chained hydrophobic cations to glass and plastic surfaces can render the modified surface capable of killing bacteria on contact. This method does not involve the release of antiseptics – the long-chained cations intrinsically destroy bacteria upon contact, by virtue of its structure. Unlike other surface treatments, no regeneration of the active material is required, aside from clearing debris from the hydrophobic cations, which can simply be done by washing with detergent. A wide variety of surfaces and textiles can be rendered bactericidal this way. As an added benefit, it appears that bacteria will have little opportunity to adapt against this threat. By directly disrupting the cell wall, this treatment avoids the biochemical route which often leads to resistance. Furthermore these polymers lack any close natural analogs, indicating that resistance development will be very unlikely [7].
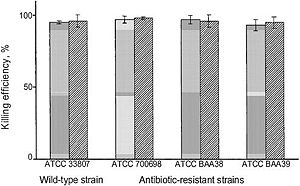
In a study by Lin et al., poly(vinyl-N-hexylpyridinium) chains were found “equally lethal against wild-type and mutant, including antibiotic-resistant, strains of the ubiquitous pathogenic bacterium Staphylococcus aureus,” killing between 90-99% of the S. aureus bacteria. [1] This was measured by exposing both airborne and waterborne bacteria to hexy-PVP coated polyethylene slides. The airborne microbes were sprayed onto the surface, while the waterborne microbes were suspended in solution and immersed with the surface with periodic shaking. [1] These hexyl-PVP chains kill a wide variety of both Gram-positive and Gram-negative bacteria, and the activity of the surface can be reset simply by washing it with detergent [1]. A wide variety of multidrug resistant (MDR) bacterial strains were also destroyed by exposure to hexy-PVP. As seen in figure 1, there appears to be little distinction whether the strain was drug-resistant or not - both wild-type and MDR strains were destroyed with exceeding aptitude. This suggests that the mechanism of PVP bactericidal activity is entirely different from that of antibiotics. It is proposed that the chains penetrate the bacterial cell wall/ membrane, and cause lysis by severing the structures at many points. The cationic pyridine nitrogen groups - the repeated unit in each polymer - provides a net positive charge that allow the polymer to puncture the membrane. As polymer length increases, the net charge increases and rigidity is provided both by intramolecular repulsion and repulsion of adjacent cationic polymers (Fig. 2). This novel antibacterial agent has such an overarching, destructive effect on bacteria that it will be very difficult for them to adapt. Unlike the molecular structures of antibiotics, the cationic PVP chains have no biological analog - thus bacteria are not expected to easily adapt a resistance. Only one form of resistance against these molecular “daggers” is known right now, and that is conferred by MDR pumps. The NorA MDR pump of S. aureus protects the cell by expelling amphipathic cations [1]. Mutants with a knockout in the norA gene were compared to their “pump-competent parents,” exhibiting a small difference in killing efficiency of hexyl-PVP: ~99% of airborne/waterborne of pump-lacking mutants were killed, while ~95% of airborne and ~97% of waterborne pump-competent cells were killed [1]. It is believed that the extracellular protrusion of MDR pumps aid in keeping the bacteria at “an arms length” away from the hexyl-PVP antiseptic, rather than specifically binding and expelling hexyl-PVP. It therefore seems unlikely that resistance to surface hexyl-PVP will come to fruition through MDR pumps. Surfaces such as plastic and glass could be made permanently antibacterial after being covalently bound to hexyl-PVP. This presents a wide set of possibilities for limiting bacterial growth in both indoor and outdoor environments. These surfaces seem capable for a variety of uses, especially since it has activity against both waterborne and airborne bacteria (Fig. 1). Water treatment is a possible use for this sort of material, particularly since very little (if any) agent will dissociate into solution. Hypothetically, even floors in high-traffic areas such as hospitals could be given this treatment, radically decreasing the amount of bacteria in the hospital. Furthermore, all manner of intracutaneous devices can be treated this way to prevent post-surgery sepsis – many deaths occur from bacteria embedded on such devices during insertion into the human body.
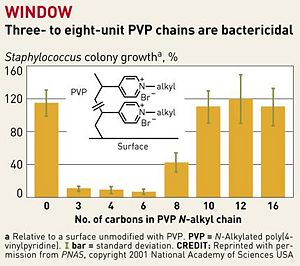
Previous attempts to design antibacterial surfaces in this manner were largely unsuccessful, because the “polymer chains weren't sufficiently long and flexible to penetrate bacterial cell walls” [8]. The addition of a linker portion to extend the N-alkylated pyridine groups and a “sweet spot” range of 3-8 PVP chains per unit has fixed this problem (Fig. 2). As seen in figure 2, chains that are either shorter or longer than this range have significantly decreased bactericidal action. Chains that are too short are presumably unable to penetrate the plasma membrane. On the other hand, excessively long chains are overly flexible, and are not rigid enough to be guided through the membrane. The 3-8 unit hydrophobic chains are produced with enough positive charge so that they repel each other while staying flexible, so that they are guided into bacterial cell walls when in close proximity [8]. As mentioned previously, intramolecular repulsion of the cationic groups also provides rigidity to the polymers, although this effect is apparently negated when the polymer is too long.
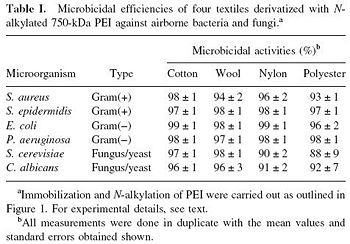
Hydrophobic polycations can also be covalently bound to porous materials such as cotton, wool, nylon, and polyester. Like hexyl-PVP, N-hexylated+methylated highmolecular-weight polyethylenimine (PEI) has strong antibacterial activity against both Gram-positive and Gram-negative bacteria [6]. A variety of microbes have been shown to perish on different kinds of PEI-laden textiles, such as C. albicans, P. aeruginosa, E. coli, and S. aureus (Fig. 3). This was tested by spraying solution-suspended bacteria and fungus/yeast onto the various textile surfaces. The surfaces themselves were prepared by covalently immobilizing PEIs through the following steps: 1. Acylation of hydrolyzed hydroxyl or amino groups of textile with 4-bromobutyryl chloride; 2. Covalent attachment of PEI; and 3. Processing of PEI to produce N-hexyl-PEI. [6] On top of the widespread antibacterial activity, textiles that are covalently bound with N-alkylated PEIs have anti-fungal (airborne) activity. This technique would be particularly useful for producing bactericidal clothes. Hospitals could be rendered bacteria-free by bedsheets and clothing that actively destroy even the most resilient multi-drug resistant bacteria. It could also be rendered a valuable defense against bioterrorism, as surmised by Lin et al. [6]. A possible limitation with this technique is that N-hexyl_PEI may "fall off" of clothing through washing and wear. In order to test continued the continued activity of antibacterial textiles, derivatized cotton was subject to simulated "laundry cycles". After two separate washes that "that included stirring overnight in soapy, warm water, followed by thorough rinsing with distilled water," the antibacterial efficiency of the large (750 kDa) N-hexyl-PEIs were unchanged. [6] However, the smaller PEI chains (0.8 - 2 kDa) showed decreased activity per wash. For this and several other mechanistic reasons, it was determined that the heavier N-hexyl-PEI polycations were of greatest interest for clothing implementation.
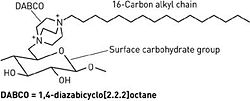
Alternative forms of alkyl chains with cationic portions are also being researched. A separate research group has devised a method for covalently attaching 1,4-diazabicyclo[2.2.2]octane (DABCO) to five different carbohydrate-based surfaces [8]. Each DABCO molecule is bound at one end to the surface carbohydrate group, and bound at the other end with a lipophilic alkyl chain of variable length (Fig 4.). The DABCO complex with a 16-carbon alkyl chain was found to have the greatest antibacterial activity, destroying both Gram-positive and Gram-negative strains. Like hexyl-PVP and N-alkylated PEIs, DABCO-hexadecane kills on contact and is permanently active [8]. It has also exhibited anti-fungal effects. Since initial testing, the DABCO complex has also been successfully bound to wool and silk.
It has not been determined whether covalently bound long-chained hydrophobic cations disrupt human cells upon contact. This is a potential concern, especially for intracutaneous devices.
Silver Nanoparticles
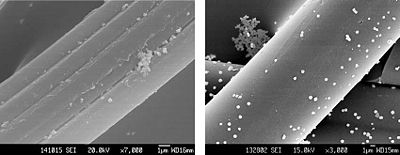
Nano-silver is an antimicrobial substance that is composed of silver particles that are on the nanometer scale. Partially oxidized nano-Ag can carry Ag+, which forms on the surface of the nano-particles [5]. Ag+ prevents DNA synthesis by directly binding to DNA [Yoshinari]. It can catalyze oxidation reactions, which in turn can disrupt proteins and cell membranes of bacteria. Ag+ interferes with membrane synthesis by forming Sulfur-Ag bonds, and by adhering to membrane proteins [Yoshinari].
When loaded with Ag through adsoption, Nano-SiO¬2 gains antibacterial properties. According to Wang et al., this antibacterial agent has a “nearly spherical structure with average particle size about 60 nm.” [2] When grafted to wool, Ag-loaded nano-SiO¬2 can destroy up to 84% of E. coli and 94% of S. aureus. [2] This manner of antibacterial surface is limited by the weak bonding between the composite particles and the wool surface. Despite this, “even after washed for 20 times, the grafted wool gave an antibacterial ratio of around 70%,” indicating that this bonding is strong enough to keep a majority of the agent associated with the surface. [2] This type of method is proposed as a way for “fabrics such as medical clothes, protective garments, and hygienic textiles” to become bio-protective [2].
Nano-SiO¬2 is a resilient structure that can also carry other inorganic antibacterial elements, such as zinc [4]. Zeolite, phosphates, apatite, and titanium oxides have also been successfully adhered to nano-SiO2. Depending on the amount of metal present, the contact time needed to kill bacteria range from 2 to 10 hours [4].
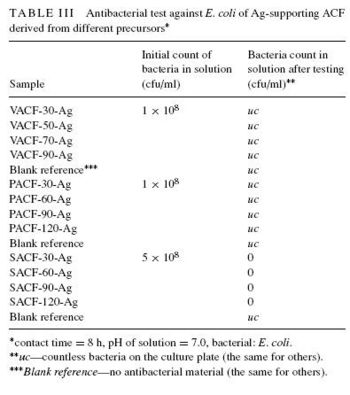
In the treatment of water, activated carbon fiber (ACF) has been used to remove pollutants via adsorption. These fibers encompass a very large surface area, and contain many pores that “collect” pollutants suspended in water. The activity of these fibers can be hampered by the growth of bacteria that adhere to the micropores. The fusing of silver with ACF represents a countermeasure against this bacterial incursion. Chen et al. examined the antibacterial activity of silver supporting activated carbon fibers (ACF-Ag), finding it extremely effective against E. coli and S. aureus [3]. Different silver-supporting activated carbon fibers exhibited antibacterial potency, although sisal based ACF (SACF) was by far the best surface, due to its porousness. (Fig. 5). The viscose and pitch based ACFs were deemed to smooth, and their hydrophobic properties made it more difficult for attached silver to interact with bacteria.[3] Therefore, bacteria cultures were "uc" (uncountable) because little VACF/PACF bactericidal activity occured. Antibacterial activity was determined to be directly related to the amount of silver adhered to ACF. Interestingly, water treatment was aided by leaving enough of the ACF specific surface area available for bacterial adsorption, bringing said bacteria into closer contact with the silver. The theory for this mechanism is supported by the higher activity of the porous SACF, as opposed to VACF or PACF. After being employed for bacterial destruction, the activity of ACF-Ag was regenerated by washing it with deionized water. After 5 cycles of washing, no reduction in ACF-Ag antibacterial activity was observed [3]. A potential concern with this method of water purification is over-exposure to suspended silver. Under the conditions of Chen et al., whereby 50 mg of ACF-Ag complex was soaked and shaken in 50 ml water for several hours, the maximum amount of silver released was around 10 ppm, meeting “the quality standard of drinking water” [3]. Most water treatment does not use this much ACF per amount of water, so it is surmised that the concentration of released silver will be even lower. Since silver can become permanently deposited in various human body tissues, it is important to keep the concentration of released silver low.
Ag-loaded nano-SiO2 also presents a potential health hazard, due to the high rate of dissociation from wool when washed. While silver is a fairly harmless element for people, nano-SiO2 structures could potentially build up in our bodies after constant exposure.
Other Methods
According to Yoshinari et al., the surface of titanium can be modified as to render it antibacterial [9]. This has potential use for providing safer titanium dental implants, particularly for ones that are constantly exposed to the oral cavity. Even though titanium surfaces are highly polished, bacteria are still capable of adhering to these implants. This treatment process including ion implantation, oxidation of the surface, and ion plating on pure titanium plates were found to “significantly inhibit the growth of both P. gingivalis and A. actinomycetemcomitans” [9]. The growth of mouse fibroblast cells was not inhibited in the presence of the modified titanium, indicating that this method is safe for human use. While fluorine ions were implanted into the titanium, no significant release of F+ was detected. The proposed antibacterial mechanism involves a “metal fluoride complex” that forms on the surface of titanium during the dry surface modification [9]. The complex appears to act on bacteria once they’ve adhered to the surface, explaining why the non-adhesive fibroblast cells continued to grow unharmed. Because relatively little F+ is released, this appears to be safe for use in vivo. Fluoride is a widely used antibacterial agent in dentistry, because released fluorine ions have an inhibitory effect on bacterial metabolic enzymes.
A study by Oyane et al. has revealed the use of immobilizing various natural antibacterial agents on the surface of an ethylene-vinyl alcohol copolymer (EVOH) [10]. Percutaneous (within-skin) devices such as catheters are now being designed to achieve maximum adhesion of the device to skin. When coated with a laminin-apatite layer, EVOH exhibits a very close association with skin, however it cannot inhibit bacteria that may be deposited on its surface [10]. The agents lactoferrin, tetracycline, and gatifloxacin were chosen for their effectiveness against E. coli and S. aureus, and were successfully immobilized on the EVOH surface within the laminin-apatite layer. The method of antibacterial activity is based on release of the agent- lactoferrin, tetracycline, and gatifloxacin disassociate from the EVOH surface at varying rates. These antibiotics then proceed to inhibit bacterial growth once released. For further study, the “ideal” agent would have high efficacy against the target bacteria, coupled with a slow release rate [10]. The initial stages of percutaneous device insertion are the most sensitive to bacterial infection, where the skin has not yet formed a tight association with the device. Therefore, permanent anti-bacterial activity is not as important in this circumstance.
.
Conclusion
The subject of antibacterial surfaces encompasses an extremely wide array of ongoing research. Current strategies have yet to be tested in field conditions, so it is difficult to estimate the large-scale bactericidal effect. Three concerns arise in development of these surfaces: cost effectiveness, safety, and microbial adaptation. Given the static nature of many surfaces, it is crucial to design an active surface that microbes have difficulty adapting to.
The above examples are by no means an exhaustive list of the uses of antibacterial surfaces. If these materials are shown to have an acceptably small impact on environmental and human health, it is very likely that their use will become widespread and pervade the world of manufacturing.
References
[4] [ Jia, H., Hou, W., Wei, L., Xu, B., and Liu, X. “The structures and antibacterial properties of nano-SiO2 supported silver/zinc-silver materials”. Dental Materials. 2008. Volume 24. pg. 244-249.]
[5] [ Lok, C., Ho, C., Chen, R., He, Q., Yu, W., Sun, H., Tam, P., Chiu, J., and Che, C. “Silver nanoparticles: partial oxidation and antibacterial activities”. J. Biol. Inorg. Chem. 2007. Volume 12. p. 527-534. ]
[9] [ Yoshinari, M., Oda, Y., Kato, T., and Okuda, K. “Influence of surface modifications to titanium on antibacterial activity in vitro.” Biomaterials. 2001. Volume 22, p. 2043-2048.]
[10] [ Oyane, A., Yokoyama, Y., Uchida, M., and Ito, A. “The formation of an antibacterial agent-apatite composite coating polymer surface using a metastable calcium phosphate solution.” Biomaterials. 2006. Volume 27, p. 3295-3303.]
Edited by Charles Halsted, student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
