Aphids and Buchnera: Difference between revisions
| Line 37: | Line 37: | ||
===Mechanisms leading to endosymbiont-host interactions=== | ===Mechanisms leading to endosymbiont-host interactions=== | ||
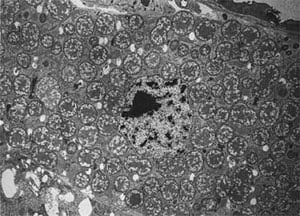
[[Image:weevil2.png|thumb|300px|right|Endosymbionts escaping out of bacteriocytes after RNAi targeting antimicrobial peptide genes. Login, F.H. et al. Science 334, 362 (2011).]] | [[Image:weevil2.png|thumb|300px|right|Endosymbionts escaping out of bacteriocytes after RNAi targeting antimicrobial peptide genes. Login, F.H. et al. Science 334, 362 (2011).]] | ||
Clues in how host-endosymbiont interaction evolved have been found in recent expression studies of insect transcriptomes, many of which | Clues in how host-endosymbiont interaction evolved have been found in recent expression studies of insect transcriptomes, many of which point toward the regulation of insect host defenses. In a recent study, RNAi targeting antimicrobial peptide genes have shown to disrupt the mutualistic relationship of weevils and their endosymbionts within bacteriocytes [[#References |[10]]]. Such discoveries provided insights of how might relationships like aphids and Buchnera be maintained and evolved. | ||
===Discovery of other endosymbionts=== | ===Discovery of other endosymbionts=== | ||
Revision as of 00:00, 6 April 2012
Introduction
Insects are one of the most diverse groups of animals. In order to fit into various types of environmental niches, many species have established mutualistic relationships with endosymbiotic bacteria for nutritional, digestive or other benefits. Aphids are sap-feeding insects infesting wide ranges of plant species. Although sap fluids from plant phloem contain high concentrations of carbohydrates, they are deficient in nitrogenous nutrients such as specific amino acids [1]. To overcome nutritional deficiency of such unbalanced diets, species within Aphidoidea have established mutualistic relationships with the obligate intracellular endosymbiont Buchnera aphidicola [2]][3]. Buchnera are grown within specialized cells known as bacteriocyes. They produce nutrients (essential amino acids) that are not synthesized by insects or obtained sufficiently from plant saps [2]. In return, aphids provide Buchnera with other nutrients required to survive [4]. Relationships between these two groups of organisms have existed dating back 150~200 millions of years ago. Due to the unique lifestyle within hosts, Buchnera have lost large numbers of genes resulting a small genome [5]. As molecular studies in this area continues, many examples of similar or more complex relationships in other organisms have been revealed, together leading to explorations of novel concepts both in the ecology and evolutionary biology.
Environment within aphid hosts
Living within bacteriocytes
Buchnera are grown in specialized cells — the bacteriocytes of aphids, and are enclosed within small vesicles derived from membranes of them. They are maternally passed to insect offspring during reproduction through the ovary and eggs [6] to ensure continuous transmission.
Nutrients in the environment
Buchnera are continuously provided with nutrients from the host, most of them including carbohydrates and nonessential amino acids (glutamate,serine, aspartate, glutamine, proline, alanine and asparagine) that are synthesized by the host or found abundant in plant saps. Such unique environment resulted in high dependence of Buchnera on their host and is reflected in the small size and composition of its genome [5]. Genome studies have shown that many of the crucial metabolic genes seen in free-living bacteria are not found in Buchnera genomes.
Host gene regulation determines environmental conditions
Unlike other environments, physical and chemical conditions within an organism could be largely determined by genetic backgrounds of the hosts. Studies in expression profiles of aphids have shown that genes involving non-essential and essential amino acid biosynthesis are up-regulated in bacteriocytes harboring Buchnera, which provide nutrients or partial biosynthetic steps of essential amino acids [7].
Microbial communities
Although Buchnera are separately grown within bacteriocytes of the aphids, other bacteria have also been found in aphids either living within bacteriocytes or not [8]. Among them include Wolbachia species and Hamiltonella defensa. The latter has be found to benefit their host by providing them protection against natural enemies [9].
Microbial processes and activities
Biosynthesis of essential amino acids
Treatments of antibiotics on aphids have shown detrimental effects in development and reproduction, which could be complemented by supplementation of crucial amino acids in diets. Together with findings showing retention of amino acids biosynthesis genes in the small Buchnera genome, it was concluded that Buchnera benefit their host mainly by producing essential amino acids[5][2].
Utilization of nutrients in the host
Due to the genome reduction process during evolution, Buchnera have lost genes necessary to produce many nutrients [5]. For example, in order to produce essential amino acids, the bacteria requires nitrogenous substrate provided by their host. These include nonessential amino acids such as serine, aspartate, glutamate, and glutamine.
Genomic characteristics of Buchnera correspond to their unique lifestyle
Unlike many microbes and pathogens living in animals, Buchnera do not harbor genes necessary for cell-surface components. Genes involving lipopolysaccharide and phospholipid biosynthesis are lacking in Buchnera genomes. Also, regulator and defense genes that are crucial for free-living bacteria are not found [5], together making them unable to survive outside of their host. Such characteristics are crucial for this organism to establish symbiotic relationships with their hosts.
Current Research
Mechanisms leading to endosymbiont-host interactions
Clues in how host-endosymbiont interaction evolved have been found in recent expression studies of insect transcriptomes, many of which point toward the regulation of insect host defenses. In a recent study, RNAi targeting antimicrobial peptide genes have shown to disrupt the mutualistic relationship of weevils and their endosymbionts within bacteriocytes [10]. Such discoveries provided insights of how might relationships like aphids and Buchnera be maintained and evolved.
Discovery of other endosymbionts
Since the first genome study of Buchnera [5], sequencing technologies have continued to improve dramatically. Endosymbionts similar to Buchnera are thus being discovered or sequenced in many other animals, some of which showing more extreme adaptations toward symbiotic lifestyles. For example, the endosymbiont Carsonella rudii found in Pachpsylla venusta, was shown to have a genome size as small as 160 kilobases [11].
Evolution of organelles
Obligate endosymbiont like Buchnera share various characteristics with organelles found in eukaryotes [12]. Unique relationships of these endosymbionts with hosts have been served as important models in understanding the evolution process of endosymbiosis and eventually the origin of organelles [13].
References
1. Sandström, J., and Moran, N.A., How nutritionally imbalanced is phloem sap for aphids? Entomol. Exp. Appl., 1999. 91(1): p. 203-10.
2. Lai, C.Y., Baumann, L., and Baumann, P., Amplification of trpEG: adaptation of Buchnera aphidicola to an endosymbiotic association with aphids. Proc Natl Acad Sci U S A, 1994. 91(9): p. 3819-23.
3. Munson, M.A., Baumann, P., and Kinsey M.G., Buchnera gen. nov. and Buchnera aphidicola sp. nov., a Taxon Consisting of the Mycetocyte-Associated, Primary Endosymbionts of Aphids. Int J Syst Bacteriol, 1991. 41(4): p. 566-8.
4. Wilkinson T.L., and Douglas A.E., Why Pea aphids (Acyrthosiphon pisum) Lacking Symbiotic Bacteria Have Elevated Levels of the Amino Acid Glutamine. J Insect Physiol, 1995. 41(11): p. 921-7.
5. Shigenobu, S., et al., Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature, 2000. 407(6800): p. 81-6.
6. Baumann, P., Endosymbiosis of animals with plant microorganisms1965, New York: : Interscience.
7. Hansen, A.K. and Moran, N.A., Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci U S A, 2011. 108(7): p. 2849-54.
8. Gomez-Valero, L., Soriano-Navarro, M., Pérez-Brocal, V., Heddi, A., Moya, A., García-Verdugo, J.M., and Latorre, A., Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J Bacteriol, 2004. 186(19): p. 6626-33.
9. Dion, E., Polin, S.E., Simon, J., and Outreman, Y., Symbiont infection affects aphid defensive behaviors. Biol Lett, 2011. 7(5): p. 743-6.
10. Login, F.H., Balmand, S., Vallier, A., Vincent-Monégat, C., Vigneron, A., Weiss-Gayet, M., Rochat, D., and Heddi, A., Antimicrobial peptides keep insect endosymbionts under control. Science, 2011. 334(6054): p. 362-5.
11. Tamames, J., Gil, R., Latorre, A., Peretó, J., Silva, F.,J., and Moya, A., The frontier between cell and organelle: genome analysis of Candidatus Carsonella ruddii. BMC Evol Biol, 2007. 7: p. 181.
12. Andersson, J.O., Evolutionary genomics: is Buchnera a bacterium or an organelle? Curr Biol, 2000. 10(23): p. R866-8.
13. Douglas, A.E. and Raven, J.A., Genomes at the interface between bacteria and organelles. Phil. Trans. R. Soc. Lond. B, 2003. 358: p. 5-18.
Edited by <your name>, a student of Angela Kent at the University of Illinois at Urbana-Champaign.