Bacillus Calmette-Guérin therapy
Summary of the Article
By Karina Kunka
This is an overview of a type of immunotherapy used to treat superficial and early-stage bladder cancers, and to prevent recurrence thereof. This particular type of immunotherapy utilizes a strain of attenuated Mycobacterium bovis to stimulate an immune response and cause direct cytotoxicity within the bladder cancer cells. Side effects are common, but typically not severe and manageable. It is the most commonly-used form of immunotherapy and remains a treatment of choice for managing of early-stage bladder cancers, as it is generally very effective at preventing recurrence of bladder cancer when maintenance therapy is used. Bacillus Calmette-Guérin is also used for a vaccine against tuberculosis infection by Mycobacterium tuberculosis in humans, and important new implications for Bacillus Calmette-Guérin use in other types of cancer treatments are up and coming.
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: BCG_infected_cells.jpeg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Recent studies have found that the Pak1-dependent pathway activated by oncogenes is actually responsible for uptake of BCG via macropinocytosis.
[1]
[2]
[3]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Introduction
Bacillus Calmette-Guérin (BCG) Therapy (Trade name TheraCys®, TICE® BCG) (chemocare.com) is a form of outpatient intravesical immunotherapy for the treatment of bladder cancer. BCG therapy is typically used to prevent noninvasive or minimally-invasive bladder cancers from returning following transurethral resection of bladder tumor (TURBT) (American Cancer Society cancer.org) and is widely considered the most effective prophylactic treatment for patients with non-muscle-invasive bladder cancer (NMIBC) (Babjuk et al., 2011).
Bacillus Calmette-Guérin is a type of attenuated (weakened or non-virulent) Mycobacterium bovis. It was first cultured at the Pasteur Institute in the early 1900s, where it was then subcultured and distributed worldwide. BCG was first used in a vaccination for tuberculosis infection, then later in the 1980’s adopted for use in immunotherapy for treatment of bladder cancer. TICE, Armond-Frappier, and Connaught are the only three FDA-approved BCG strains for use in patients with non-muscle-invasive bladder cancer, but TICE is the only strain actively marketed in the U.S. as of 2017 (Boehm et al., 2017). However, many other strains exist and are used worldwide for the same effect (see: Efficacy).
Mycobacterium bovis
Overview
Mycobacterium bovis is a strain of aerobic, slow-growing bacteria that is the main cause of bovine tuberculosis. It is closely related to Mycobacterium tuberculosis, or the main causative agent of tuberculosis in humans, which can lead to severe infection of the lungs and lymph nodes. M. bovis typically infects cattle, deer, elk, and bison, but it is actually capable of crossing the species barrier and infecting humans with tuberculosis, though it is estimated to cause only 2% of total tuberculosis cases in the United States each year (CDC). Transmission is usually through contaminated dairy products or other products that come from host animals like meat (CDC).
M. Bovis History and Attenuation
For medical use, the virulence of M. bovis must be reduced so as to not lead to infection of the patient during BCG immunotherapy. BCG was first attenuated for use in the BCG vaccine against M. tuberculosis in 1921 by Albert Calmette and Camille Guérin in the Pasteur Institute in Paris, France (See “Other Uses of BCG”) (Boehm et al., 2017). The most common method of attenuation for a bacterial strain is adaptation to a foreign host. The pathogenic bacteria are exposed for periods of time to tissues, eggs, or live specimens of these foreign hosts and allowed to develop mutations that make them better able to survive in the new environment. These mutations make subsequent infection of humans much more difficult, and human pathogenicity of the new strain is decreased. For the creation of BCG from M. bovis, the bacterium was attenuated via serial passage of M. bovis bacteria on glycerol-enriched potatoes (Boehm et al., 2017). The mechanism of attenuation of BCG is not fully understood, as BCG is not a single strain, but rather a collection of substrains that exhibit differing levels of virulence (Liu et al., 2009). However, it is known that attenuated BCG strains contain mutants of several virulence factors of mycobacterium tuberculosis, such as ESX-1 (a virulence factor secretory system), PDIM/PGL (cell envelope localized Mycobacterium tuberculosis lipids and related glycolipids), and PhoP (a transcriptional regulatory protein), making these mutations important for lack of BCG pathogenesis (Liu et al., 2009).
Bladder Cancer
Ranking fourth, bladder cancer is one of the most common types of cancer in men. It was predicted that in the year 2016, almost 77,000 new cases of bladder cancer were responsible for over 16,000 deaths in the U.S. alone (Cookson et al, 1997). Most cases of bladder cancer present as NMIBC, which includes stage Ta (non-invasive papillary carcinoma) and CIS (Carcinoma in situ: non-invasive flat carcinoma) which makes up stage 0, and T1(tumor has grown from bladder lining to connective tissue, but has not yet reached the muscle layer) which makes up stage 1 bladder cancer. For these early stages, standard treatment involves removal of papillary lesions with transurethral resection. However, recurrence rates are high even when it appears as if all cancerous tissue is removed, and secondary treatments in the form of BCG therapy are often used (Pagano et al., 1991).
Delivery and Course of Treatment
BCG therapy is delivered via an intravesicular infusion, meaning that a catheter is inserted into the urethra, and BCG solution is injected directly into the bladder via the catheter, which is then clamped to prevent BCG solution from prematurely exiting the bladder for the duration of the catheterization. The patient receiving BCG treatment must roll side to side and lay in multiple positions in order to guarantee the medication reaches all areas of the bladder. After about 2 hours, the clamped is removed and the fluid is drained, then the catheter is removed. Typically this is repeated on a weekly basis for 6 weeks following TURBT, then maintenance treatment is repeated monthly for a predetermined time by the physician based on risk of recurrence (chemocare.com). Current guidelines suggest 1-3 years of maintenance of BCG therapy following TURBT for reducing the chance of recurrence or progression into more aggressive, muscle-invasive forms of bladder cancer (Babjuk et al., 2011). The amount of BCG administered per treatment depends on the manufacturer, the health of the patient, and the cancer type as evaluated by a physician.
Efficacy
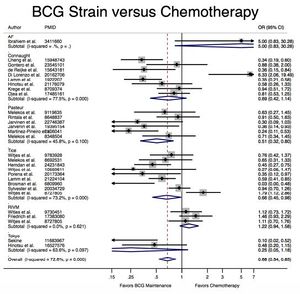
A meta-analysis of 65 studies with a total of 12,246 patients presenting with early-stage bladder cancer receiving Bacillus Calmette-Guérin immunotherapy treatment from 1978-2016 revealed that BCG significantly reduced cancer recurrence when compared with chemotherapy and surgery alone (Boehm et al., 2017). Recurrence rates varied by strain; for example, the Connaught strain had a 74% 5-year recurrence-free survival rate as compared with the 48% of the TICE strain, and was generally more effective when no maintenance treatments were administered. However, the TICE strain was more effective than the Connaught strain for the time to first recurrence when no BCG maintenance therapy was given. Lack of head-to-head studies makes ranking efficacy difficult, but based on Surface Under the Cumulative Ranking Curve (SUCRA values), the Tokyo, Pasteur, and TICE strains appear to rank 1st, 2nd, and 3rd in efficacy respectively, though no definitive statements can be made (Boehm et al., 2017).
Mechanism of Action
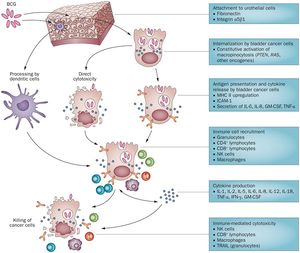
(Subheading) Overview The complete mechanism of action for Bacillus Calmette-Guérin therapy is not fully known, but BCG can be classified as a biological response modifier. This means that the treatment stimulates the immune system to attack the bladder cancer cells, rather than having some sort of primary antitumor capabilities (chemocare). Currently, Bacillus Calmette-Guérin immunotherapy is thought to be effective through a variety of mechanisms. (Subheading) Cells involved Research suggests that the mechanism of BCG’s antitumor effects involves cells of the immune system and urothelial cells, which includes both cells lining the bladder and the bladder cancer cells themselves. The cancer cells must attach and internalize the BCG cells, secrete cytokines and chemokines, and express BCG antigens for localization by immune cells. Immune cells currently thought to play a role in BCG’s antitumor effects include CD4 and CD8 lymphocytes, as well as many others including dendritic cells, macrophages, granulocytes, and natural killer cells. These immune cells secrete soluble factors to induce direct cytotoxicity such as TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) to kill theBCG- infected bladder cells. The direct action of BCG (i.e. cancer cell lysis as a result of internalization of BCG) is also thought to play a role in antitumor effects, if only to some smaller degree (Redelman-Sidi et al., 2014). (Subheading) Uptake of BCG by Bladder Cancer Cells It would be expected for bladder cancer cells to uptake BCG through phagocytosis, but recent studies have found that the Pak1-dependent pathway activated by oncogenes is actually responsible for uptake of BCG via macropinocytosis (Redelman-Sidi et al., 2013). Redelman-Sidi et al. determined that cells vary drastically in their ability to uptake BCG, and bladder cancer cells’ use of micropinocytosis to internalize BCG may account for some of this variability. There are two resulting classes of bladder cancer cells: the Bacillus Calmette-Guérin permissive and resistant cells, with the BCG-permissive cells having a much higher degree of BCG uptake than the resistant phenotype (Redelman-Sidi et al., 2013).The proteins responsible for uptake of BCG into bladder cancer cells are Rac1, Cdc42, and Pak1 as an effector kinase (Redelman-Sidi et al., 2013). Differences in susceptibility of BCG passive versus resistant cells was found to be caused by oncogenic activation of the signaling pathways responsible for the activation of micropinocytosis (Redelman-Sidi et al., 2013). The phosphoinositide 3-kinase inhibitor stimulates GCB uptake independently of Akt. Activated Ras protein also activated Pak1-dependent uptake of BCG (Redelman-Sidi et al., 2013). This means that the activation of micropinocytosis by oncogenes determines the degree of BCG uptake by urothelial cancer cells. Furthermore, this may also imply that antitumor effects of BCG may be governed by specific mutations affecting the pathways stated above that are present in the BCG-treated cancer cells. In effect, BCG therapy relies on uptake mechanisms tied to the same mutations that lead to the formation of these cancer cells, meaning that it exploits this property specific to bladder cancer cells to induce an immune response that attacks the cancer instead of normal urothelial cells (Redelman-Sidi et al., 2013). (Subheading) Step-by-step overview 1) Attachment to the urothelial cells is accomplished through the use of fibronectin and integrin α5β1. The bladder cancer cells internalize BCG through constitutive activation of micropinocytosis though oncogenes such as PTEN (Phosphatase and tensin homolog) which acts as a tumor suppressor when functional, RAS (a small GTPase protein involved in cellular signal transduction ), and others (Redelman-Sidi et al., 2014). 2) Once BCG cells are internalized, they cause either direct cytotoxicity or stimulate presentation of cytokines and antigens. MCH II (a class II antibody) is upregulated, ICAM-1(Intercellular Adhesion Molecule 1) is produced, and interleukin (IL) 6 & 8, GM-CSF (granulocyte macrophage colony-stimulating factor), and TNF- α (Tumor Necrosis Factor-α) are secreted (Redelman-Sidi et al., 2014). 3) Bladder cancer cell disruption via direct cytotoxicity or cytokine/antigen presentation results in recruitment of immune cells such as granulocytes, CD4 lymphocytes, CD8 lymphocytes, natural killer cells, and macrophages. The cytokines produced in this process of immune cell recruitment include IL-1, IL-2, Il-5, IL-6, IL-8, IL-12, IL-18, TMF- α, IFN-γ (interferon-γ), and GM-CSF (Redelman-Sidi et al., 2014). 4) Immune cell recruitment and cytokine production ultimately results in immune-mediated cytotoxicity by natural killer cells, CD8 lymphocytes, and macrophages. Tumor necrosis factor-related apoptosis-inducing ligand is also secreted by granulocytes to break down the cancer cells (Redelman-Sidi et al., 2014). It is also important to note that not all BCG is taken up by bladder cancer cells. While some BCG is internalized by cancer cells causing cytotoxicity and stimulating immune responses, other BCG cells are broken down by dendritic cells that help protect normal urothelial cells from BCG infection (Redelman-Sidi et al., 2014).
Complications of BCG Therapy
Conclusion
References
- ↑ Redelman-Sidi et al., "Oncogenic Activation of Pak1-Dependent Pathway of Macropinocytosis Determines BCG Entry into Bladder Cancer Cells." 2013. Cancer Research 73:3 1156-1167.
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
