Bacillus cereus: Difference between revisions
| Line 119: | Line 119: | ||
'''8.1 Biofilms of ''B. cereus''''' | '''8.1 Biofilms of ''B. cereus''''' | ||
The ability of ''B. cereus'' to form biofilms on surfaces can cause potential contamination problems within the food industry. Therefore, biofilm formation of several ''B. cereus | The ability of ''B. cereus'' to form biofilms on surfaces can cause potential contamination problems within the food industry. Therefore, biofilm formation of several ''B. cereus'' strains are currently being researched to prevent potential food contamination and to ensure safety during production. In a recent study, microtiter assay and assays on stainless steel were completely or partially submerged in liquid in order to observe ''B. cereus'' biofilm formation. Since stainless steal is commonly used for pipes and tanks in the food industry, additional tests were conducted to investigate ''B. cereus'' biofilm formation from spores on stainless steal coupons. The results from both tests were similar. It appears that ''B. cereus'' biofilms preferentially form within the air-liquid interface. This tendency is due to the availability of oxygen in this region, causing ''B. cereus'' to move toward oxygen. In addition, spore formation was more rapid in the suspension phase of biofilm formation, suggesting that biofilms are a cavity for sporulation. The results show that ''B. cereus'' biofilms may develop within storage and piping systems when either partially filled or when liquid residues remain during production. In addition, increase in spore formation by ''B. cereus'' within biofilms can potentially cause recontamination and equipment failure during food production [29]. | ||
'''8.2 Effects of Procine Bile on ''B. cereus''''' | '''8.2 Effects of Procine Bile on ''B. cereus''''' | ||
Revision as of 19:55, 27 August 2007
A Microbial Biorealm page on the genus Bacillus cereus
Classification
===Higher order taxa===
Domain: Bacteria
Phylum: Firmicutes
Class: Bacilli
Order: Bacillales
Family: Bacillaceae
Genus: Bacillus
Species Group: Bacillus cereus group
Species
|
NCBI: Taxonomy |
Bacillus cereus
Description and significance
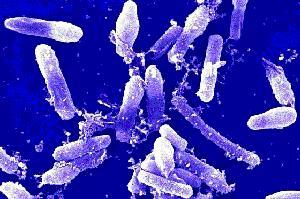
Bacillus cereus is a large, 1 x 3-4 µm, gram-positive, rod-shaped, endospore forming, facultative aerobic bacterium [2]. It was first successfully isolated in 1969 from a case of fatal pneumonia in a male patient and was cultured from the blood and pleural fluid [5]. 16s rRNA comparison reveals Bacillus cereus to be most related to Bacillus anthracis, the cause of anthrax, and Bacillus thuringiensis, an insect pathogen used as pesticide [3]. Although they have similar characteristics they are distinguishable with B. cereus being most motile, B. thuringiensis produce crystal toxins, B. anthracis is nonhemolytic [4].
B. cereus is a mesophile, growing optimally at temperatures between 20 and 40°C, and is capable of adapting to a wide range of environmental conditions. It is distributed widely in nature where it is most commonly found in the soil as a saprophytic organism. B. cereus is also an inhabitant of the microflora of insects as a gut commensal where it derives its nutrients from its host, and is found in the rhizosphere of some plants [2]. B. cereus spores and cells are released back into the soil where they germinate upon defecation or death of their host until they are ingested by another host.
As a soil bacterium, B. cereus can spread easily to many types of foods such as meat, eggs, dairy products, and plants, and is commonly known for causing 25 % of food-borne intoxications due to is production of emetic toxin and enterotoxins [4]. Food poisoning occurs when food is left without refrigeration for several hours before it is served. Surviving spores of contaminated foods after heat treatment grows well and is the source of food poisoning [4].
In addition, Bacillus cereus is an opportunistic human pathogen and is occasionally associated with infections, causing periodontal diseases and other more serious infections [5]. Immunocompromised individuals are susceptible to bacteremia, endocarditis, meningitis, pneumonia, and endophthalmitis [6]. Its potential to cause systemic infections are of current bio-medical and public health concerns. Thus, the genome sequence of Bacillus cereus is significant in establishing genetic background information for further investigations and to promote public health preparedness. Sequencing its genome is vital in order to expand the understanding of its pathogenicity, and for the development of anti-microbial drugs. Additionally, since Bacillus cereus strains are so genetically closely related to B. anthracis, used in biological warfare, and can display anthrax-like virulence traits, genomic comparisons between the two species is important in order to study the virulence of B. anthracis to produce vaccines and prevent its usage as a bioweapon.
Genome structure
3.1 Genome
B. cereus has a circular chromosome measuring 5,411,809 nt in length and was completely sequenced using the shotgun sequencing method [7]. The genome structure of B. cereus consists of 5481 genes, 5234 protein codings, 147 structural RNAs, and 5, 366 RNA operons [1]. An interesting gene cluster found within its genome encodes for arginine deiminase metabolic pathway. The presence of this cluster is predicted to have a role in its survival, enabling B. cereus to be resistant to acidic conditions in a similar manner Streptococcus pyogenes does [8]. Additionally, B. cereus has a nine gene urease gene cluster that encodes for proteins, blasticidin S deaminase , and an S-layer protein [8]. The urease enzyme has a role in increasing vigor of B.cereus in acidic conditions, and is similar to the Helicobacter pylori urease required for colonization of the human stomach [8].
Genes present within the chromosome associated with B. cereus virulence include non-haemolytic enterotoxins, channel-forming type III hemolysins, phospholipase C, a perfringolysin O (listeriolysin O), and extracellular proteases [6]. The hbl operon, an RNA transcript of 5.5 kb, transcribes all three proteins of the hemolysin BL enterotoxins associated with food poisoning. These genes along with other genes encoding for metabolic enzymes, proteins involved in motility and chemotaxis, proteins involved in sporulation, and cellular transporters are all regulated by the plcR gene [9]. In addition to its role in the regulation of transcription of these genes for survival, the plcR gene is required for the full virulence of B. cereus. Therefore, this gene is often the target of antimicrobial agents. Another gene found on its chromosome is the gerA gene which is essential for the sporulation of B. cereus when nutrients are depleting, and is responsible for spore germination in response to l-alanine and ribosides [9].
B. cereus have fewer genes for carbohydrate catabolism, with only 12 carbohydrate polymer degradation genes, and more genes for amino acid metabolism. Therefore, B. cereus is more susceptible to environments that are rich in protein. Furthermore, B. cereus has an 18-23 gene that encode peptide and amino acid ABC transporter-ATP binding proteins which suggests that proteins, peptides, and amino acids are preferred nutrient sources [9].
3.2 Plasmids
B. cereus has a diverse range of plasmids that vary in size from 5 to 500 kb, and is known to have more than one plasmid with only a few that are associated with pathogenesis. B. cereus ATCC has a large pXO1 plasmid that is also found in B. anthracis [7]. However, it is avirulent because it does not have the portion that encodes for the toxin and regulatory proteins. B. cereus G9241 has a plasmid that is 99.6% identical to pXO1 plasmid from B. anthracis, but does not have the pXO2 plasmid. It also has an extra plasmid that encodes for a capsule biosynthesis operon [7]. B. cereus ZK, a pathogenic strain has five plasmids, the largest amount of plasmids found in a B. cereus strain. Transposase genes are found in the two large plasmids which function in gene exchange between plasmids and chromosome [7]. The three smaller plasmids of the five are believed to function in the identification of replication and mobilization proteins [7].
Cell structure and metabolism
4.1 Cellular Structure
Bacillus cereus is a 1 x 3-4 µm, rod shaped gram- positive bacterium. Its cell structure consists of an inner membrane and a thick peptidoglycan which functions to maintain cell shape [10]. The polysaccharide portion makes up 50% percent of the cell wall and consists of a neutral polysaccharide composed of N-acetylglucosamine, N-acetylmannosamine (ManNac), N-acetylgalactosamine and glucose in a molar ratio of 4: 1 : 1 : 1 [11]. The acidic portion of the cell wall is characteristic in having a repeating tetrasaccharide unit within the sn-glycerol 1-phospahte residue [11]. 5% of the cell wall is made up of techoic acids consiting of N-acetylglucosamine, galactose, glycerol, and phosphorus in a molar ratio of 1 : 1.4: 1 : 1 [11]. The linkage between the polysaccharide and peptidoglycan is a muramic acid 6-phosphate [11]. The peptidoglycan of some B. cereus strains are unique with only a few oligomers present, the cross-linked muropeptides are dimmers, and many of the muropeptide lack the N-acetly group from glucosamine residues [12]. These distinguishing features affect cell surface charge which contributes to the attachment of an outer capsule or an S-layer in pathogenic strains [12].
Clinical isolates of B. cereus have a glycoprotein S-layer overlying its peptidoglycan. The S-layer consists of proteinaceous paracrystalline arrays which completely cover the cell surface. The S-layer is involved in the virulence of B. cereus, and functions to promote interactions with human polymorphonuclear leucocytes. It also enables B. cereus to adhere to type I collagen, laminin, fibronectin and fibrinogen of the epithelium, and thus has a role in increasing interaction between B.cereus and its host [13]. In addition, this proteinaceous layer contributes to its ability to thrive under harsh conditions by enhancing its resistance to radiation.
B. cereus is motile by means of flagella and exhibits two types of motility including swimming and swarming. The type of motility is dependent on the environment, whether within a liquid or solid. Single cells exhibits swimming motility by means of short flagellated rods [14]. On the other hand, swarming, which is induced by surface sensing, is a collective movement of cells involving swarm cells with flagellum that is observed to be three to four times longer and also forty times more flagellated than single swimming cells. Flagella in B. cereus play an important role in motility which is necessary for survival in order to find nutrients and to colonize surfaces within its environment. However little is known about its role in B. cereus pathogenicity.
4.2 Spore Structure
B. cereus spore formation occurs when nutrients are scarce within the environment and germinate into vegetative cells once they are available [6]. Therefore, spore structure is important to the survival of this bacterium. B. cereus spores consist of an inner core surrounded by the inner membrane, and outer cortex surrounded by the outer membrane with an additional exterior coat [16]. The spore coat is made of proteins and small amounts of lipids and carbohydrates which contributing to its resistance to oxidizing agents and chemicals by blocking toxic molecules [15]. In addition, the mineralization of spores allows them to be heat and γ-radiation resistant [15]. Germination of spores is stimulated by several factors involving nutrient and chemical triggers. Growth is in response to l-alanine and ribosides which triggers a sequence of germination events including water uptake, loss of Ca2+ and dipicolinic acid, and core metabolism [16]. Once spores begin to germinate, they are no longer resistant to heat and γ-radiation.
4.3 Metabolism
B. cereus is a facultative aerobe so it can utilize oxygen as a terminal electron accepter, but also has methods of anaerobic respiration as a mechanism of energy release. A few metabolic enzymes used by B. cereus include NADH dehydrogenases, succinate dehydrogenase, complex III, non-proton-pumping cytochrome bd quinol oxidases, proton-pumping terminal oxidases such as cytochrome, quinol oxidase, and cytochrome c oxidase [17].
Aerobic and anaerobic catabolism have different pathways in B. cereus. In aerobic respiration, reducing equivalents produced from glycolysis and the Krebs cycle are reoxidized by the electron transport chain, creating a proton motive force and ATP by ATP synthase [17]. Under anaerobic respiration, B. cereus utilize fermentation to generate energy. The TCA cyle is used minimally to produce anabolic precursors and ATP is generated by substrate level phosphorylation. Fermenation recycles NAD+ from the reducation of pyruvate and create lactate and ethanol as end products [17]. These two means of catabolism are regulated by the cytoplasmic response regulator, ResD, and ResE which sense the availability of O2 and nitrate [17].
B. cereus can metabolize a variety of compounds including carbohydrates, proteins, peptides and amino acids for growth and energy. During anaerobic respiration some, of the major products produced from carbon sources such as sucrose or glucose include L-lactate, acetate, and formate, succinate, ethanol, and CO2 [18]. During nitrate respiration, nitrate reductase converts nitrate into nitrite, and nitrite reductase converts nitrite into ammonium.
Ecology
Interactions between B. cereus and other mircroorganisms occur in the rhizosphere, the area surrounding plant roots. Plants benefit from the presence of B. cereus in the rhizosphere since they are capable of inhibiting plant disease caused by protest pathogens and also enhances plant growth [22]. B. cereus naturally produces the antibiotics zwittermicin A. and Kanosamine which are highly inhibitory to the growth of plant pathogenic oomycetes, certain fungi, and few bacterial species [19]. In addition, B. cereus increases nodulation of soybean plants by Bradyrhizobium japonicum [22].
In addition, the bacteria Cytophaga-Flavobacterium group (CF) benefit from B. cereus in the rhizosphere. CF bacteria utilize the peptidoglycan of B. cereus as a carbon and energy source by hydrolyzing the outer layer [21]. The relationship between the two organism is commensal since the growth of B.cereus is not affected by the presence of CF bacteria.
B. cereus is also found in the gut microflora of invertebrate and digestive tracts of sow bugs, roaches and termites where it exhibits filamentous growth as an intestinal symbiont of arthropods [22]. Arthropods consume feces or soil with spores or cells of B. cereus. This intestinal stage of B. cereus, also known as the Arthromitus stage, involves the attachment of endospore fibers released from a parent cell. After attachment, they begin motile and filament growth stages and attach to the epithelium [22]. B. cereus cells are then defecated back into the soil where they can continue to grow. [22]
B cereus is widely known to affect humans by causing food poisoning. Since it is ubiquitous in food products, where small amounts are inevitably consumed, this bacterium is also a contributor to the human intestinal flora [23]. Additionally, B. cereus affect humans as an opportunistic pathogen. Refer to the pathology section for further elaboration.
Pathology
Bacillus cereus causes two types of food poisoning in humans including diarrhoeal syndrome and emetic syndrome. Food poisoning results from the presence of enterotoxins produced by B. cereus in the gastrointestinal tract. The dosage of ingested B. cereus spores leading to diarrhoeal syndrome is 105–107 g 1 of ingested food, and 105–108 g 1 of ingested food for emetic syndrome [6]. Enterotoxins associated with diarrhoeal syndrome are unresistent to the acidic conditions of the stomach. However, the cereulide peptide toxin, associated with emetic syndrome, is more resistant to extreme pH values and remains active at 121 °C. Therefore, cereulide peptide toxins can continue to survive, germinate, grow, and secrete enterotoxins in the small intestine [6].
Virulence factors associated with diarrhoeal syndrome involve three enterotoxins including hemolysin BL (HBL), non-hemolytic enterotoxin (NHE), and cytotoxin K [24]. HBL is made of three proteins B, L1, and L2 and is the main virulence factor of B. cereus. Symptoms of diarrhoeal syndrome include watery diarrhea, abdominal cramps, and pain occurring 6-15 hours after ingestion, and may persist for twenty-four hours [4].
The emetic syndrome is caused by cereulide peptide toxin which is sescreted during stationary phase [25]. This toxin has a ring structure, dodecadepsipeptide, which consists of four amino acids, repeating three times, and oxy acids [25]. Symptoms associated with emetic syndrome include nausea and vomiting within half an hour to six hours after ingestion of food and also lasts for about twenty-four hours [6].
Although B. cereus is commonly known to cause food-borne intoxications, it has been reported to cause local and systemic infections, as an opportunistic pathogen, especially among immunologically compromised patients, newborns, and patients with surgical wounds or catheters. B. cereus can cause ocular infections such as keratitis, endophthalmitis, and panophthalmitis [24]. The main virulence factor in B. cereus endophthalmitis is HBL which can result in the detachment of the retina and blindness. In addition, B. cereus can cause gangrene, bovine mastitis, pyogenic infections, cellulitis, infant death,septic meningitis, periodontal disease, lung abscesses,and endocarditis [6]. However, these infections are less common.
Virulence factors associated with non gastrointestinal infections include hemolysins and phospholipase C. Hemolysin III causes erythrocyte lysis [6]. Phospholipase C causes tissue damage by inducing the degranulation of human neutrophil [24]. There are three different types of phospholipase C produced by B. cereus including phosphatidylinositol hydrolase, phosphatidylcholine hydrolase, and hemolytic sphingomyelinase [24]. Phospholipase C degrades the subepithelial matrix affecting the healing of epithelial tissue of infections.
Application to Biotechnology
7.1 Biological Control Agent
Biological control agents are alternatives to chemical pesticides that are capable of suppressing plant pests and can also enhance plant growth. Most strains of Bacillus cereus produce toxins known to cause food-borne illnesses. However, enterotoxin deficient member strains do not produce the hemolysin BL (HBL) enterotoxin. Thus, antifungal compounds of Bacillus cereus strains have been developed as a useful biological control agent in the suppression of fungi and crop disease.
7.2 Bacillus cereus Strains
Bacillus cereus B4 is used as a pesticide to deter certain fungi from rotting seedling plants. This strain produces three types of metabolites that enable it to suppress certain plant diseases while furthering the growth of potato crop. The metabolites include Kanosamine, 3,4-dihydroxy benzoate, and 2 keto-4 methylthiobutyrate. The metabolite 3,4-DOHB improves crop strength and results in a healthier and larger root system[27].
Bacillus cereus DGA34 was isolated from the environment and is useful in fighting off fungal damping disease in field crops. It naturally produces the antibiotic zwittermicin A. by fermentation which is found in the supernantant fluid of its culture medium. This antibiotic is effective in fighting a wide range of fungal and bacterial microorganisms, and reduces symptoms of damping-off disease and root rot [26].
Bacllus cereus UW85 is used as a biocontrol agent that produces two antibiotic toxins including zwittermicin A. and antibiotic B. Bacillus cereus UW85 has a wide range of usage which includes protecting alfalfa seeds from dampening off caused by Phytophthora medicaginis, cucumber fruits from Pythium aphanidermatum, peanuts from Sclerotinia minor, and tobacco seedlings from Phytophthora nicotianae,[28].
These are just a few B. cereus strains with current United States patents as biocontrol angents for crop diseases. There are currently many Bacillus cereus strains that are still under review.
Current Research
8.1 Biofilms of B. cereus
The ability of B. cereus to form biofilms on surfaces can cause potential contamination problems within the food industry. Therefore, biofilm formation of several B. cereus strains are currently being researched to prevent potential food contamination and to ensure safety during production. In a recent study, microtiter assay and assays on stainless steel were completely or partially submerged in liquid in order to observe B. cereus biofilm formation. Since stainless steal is commonly used for pipes and tanks in the food industry, additional tests were conducted to investigate B. cereus biofilm formation from spores on stainless steal coupons. The results from both tests were similar. It appears that B. cereus biofilms preferentially form within the air-liquid interface. This tendency is due to the availability of oxygen in this region, causing B. cereus to move toward oxygen. In addition, spore formation was more rapid in the suspension phase of biofilm formation, suggesting that biofilms are a cavity for sporulation. The results show that B. cereus biofilms may develop within storage and piping systems when either partially filled or when liquid residues remain during production. In addition, increase in spore formation by B. cereus within biofilms can potentially cause recontamination and equipment failure during food production [29].
8.2 Effects of Procine Bile on B. cereus
Resistance to bile is important to the survival of B. cereus within the small intestine enabling it to proliferate and release enterotoxins. Studies have been done to test the effects of porcine bile (PB) on B. cereus and on its HBL enterotoxin in small intestine media (IM). Grastic media (GM) were prepared with food items added to the small intestine media to simulate food such as chicken as protein, peas as starch and fibre, broth, and milk for fat. B. cereus was added into each gastric media, mixed with porcine bile at different concentrations, and incubated at 37°C. Results show that the growth of B. cereus was affected by the type of food in the small intestine media which may be explained by the protective effect of different food types against porcine bile. For instance, intestine media containing pea puree had a higher protective effect against porcine bile than the milk or chicken. Proteins may have a protective effect from PB seen in IM-milk and IM-chicken. Components of food such as fibre can bind to bile salts, reducing their toxic effects on B. cereus. In addition, food components with hypocholesterolemic activities, observed by IM-pea, can sequester bile salts. Therefore, the tolerance of B. cereus to porcine bile and its ability to grow and produce toxins within the small intestine is dependent on the type of food in the small intestines and on bile concentrations [30].
8.3 Fur Gene Required for B. cereus Virulence
B. cereus has been a growing and established opportunistic human pathogen. Therefore, current research is being conducted to understand its pathogenicity in order to find potential targets for antimicrobial drugs. B. cereus fur gene, a transcriptional regulator that is responsible for bacterial iron uptake and metabolism, was shown to reduce virulence in the pathogenic fur mutant. Results in the experiment revealed a decrease in the regulation of iron uptake with three times more intercellular iron in the fur mutant than in the wild-type. Also, siderophore biosynthesis occurred even in the presence of available iron in the growth medium. The increased presence of intercellular iron resulted in a greater amount of oxidizing free radicals and in sensitivity of the fur mutant to hydrogen peroxide. When the virulence of the B. cereus fur mutant was measured in an insect model infection, the significance of iron metabolism regulation in bacterial pathogens was shown by the reduced virulence of B. cereus. However, the mutant strain was not completely avirulent due to other important regulators such as PlcR associated with B.cereus virulence. The results from this recent study give insight to the importance of the fur gene in the regulation of intracellular iron concentrations for cell growth, survival, and pathogenesis. The reduced virulence of B. cereus fur mutants in this experiment demonstrates the potential of the fur gene to be a good target for antimicrobial drugs since it is a conserved protein among pathogenic bacteria [31].
References
[1] "Bacillus cereus." NCBI website. Accessed on August 18, 2007.
[2] Vilain, S., Luo, Y., Hildreth, M., and Brozel, V. “Analysis of the Life Cycle of the Soil Saprophyte Bacillus cereus in Liquid Soil Extract and in Soil.” Applied Environmental Microbiology. 2006. Volume 72(7). p. 4970–4977.
[3] DelVecchio, V., Connolly, J., Alefantis, T., Walz, A., Quan, M., Patra, G., Ashton, J., Whittington, J., Chafin, R., Liang, X., Grewal, P., Khan, A., and Mujer C. “Proteomic Profiling and Identification of Immunodominant Spore Antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis.” Applied Environmental Microbiology. 2006. Volume 72(9). p. 6355–6363.
[4] “Bacillus cereus.” United States Food and Drug Administration, Center for food safety and applied nutrition (FDA). Accessed August 18, 2007.
[5] Hoffmaster, A., Hill, K., Gee, J., Marston, C., De, B., Popovic, T., Sue, D., Wilkins, P., Avashia, S., Drumgoole, R., Helma, C., Ticknor, L., Okinaka, R., and Jackson, J. “Characterization of Bacillus cereus Isolates Associated with Fatal Pneumonias: Strains Are Closely Related to Bacillus anthracis and Harbor B. anthracis Virulence.” Journal of Clinical Microbiology. 2006. Volume 44(9). p. 3352-3360.
[6] Wijnands, L., Dufrenne, J., Zwietering, M. H., and Leusden, F. “Spores from mesophilic Bacillus cereus strains germinate better and grow faster in simulated gastro-intestinal conditions than spores from psychrotrophic strains.” International Journal of Food Microbiology. 2006. Volume 112. Issue 2. p. 120-128.
[7] Rasko, D., Altherr, M., Han, C., and Ravel, J. “Genomics of the Bacillus cereus group of organisms.” FEMS Microbiology Reviews. 2005. Volume 29(2). p.303-329.
[8] Rasko, D., Ravel, J., Okstad, O. A., Helgason, E., Cer, R., Jiang, L., Shores, K. A., Fouts, D., Tourasse, N., Angiuoli, S., Kolonay, J., Nelson, W., Kolsto, A, Fraser, C., and Read, T. D. “The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1” Nucleic Acids Research. 2004. Volume 32(3). p. 977–988.
[9] Han, C., Xie, g., Challacombe, J., Altherr, M., Bhotika, S., Bruce, D., Campbell, D., Campbell, M., Chen, J., Chertkov, O., Cleland, C., Dimitrijevic, M., Doggett, N., Fawcett, J., Glavina, T., Goodwin, L., Hill, K., Hitchcock, P., Jackson, P., Keim, P., Kewalramani, A., Longmire, J., Lucas, S., Malfatti, S., McMurry, K., Meincke, L. J., Misra, M., Moseman, B. L., Mundt, M., Munk, C., Okinaka, R. T., Parson-Quintana, B., Reilly, L. P., Richardson, P., Robinson, D. L., Rubin, E., Saunders, E., Tapia, R., Tesmer, J. G., Thayer, N., Thompson, L. S., Tice, H., Ticknor, L., Wills, P., Brettin, T., and Gilna, P. “Pathogenomic Sequence Analysis of Bacillus cereus and Bacillus thuringiensis Isolates Closely Related to Bacillus anthracis.” Journal of Bacteriology. 2006. Volume 188(900). p. 3382–3390.
[10] Ticknor, O., Kolsto, A., Hill, K., Keim. P., Laker, M., Tonks, M., and Jackson, P. “Fluorescent Amplified Fragment Length Polymorphism Analysis of Norwegian Bacillus cereus and Bacillus thuringiensis Soil Isolates.” Applied Environmental Microbiology. 2001. Volume 67(10). p. 4863–4873.
[11] Amano, K., Hazama, S., Akarari, Y., Ito, E. “Isolation and Characterization of Structural Components of Bacillus cereus AHU 1356 Cell Walls.” European Journal of Biochemistry. (1977). Volume 75 (2). p. 513–522.
[12] Severin, A., Tabei, K., Tomasz, A. “The structure of the cell wall peptidoglycan of Bacillus cereus RSVF1, a strain closely related to Bacillus anthracis.” Microbial Drug Resistance. 2004. Volume 10(2). p. 77-82.
[13] Mignot, T., Denis, B., Couture-Tosi, E., Kolsto, A., Mock, M., Fouet, A. “Distribution of S-layers on the surface of Bacillus cereus strains: phylogenetic origin and ecological pressure.” Environmental Microbiology. 2001. Volume 3(8). p. 493–501.
[14] Senesi, S., Celandroni, F., Salvetti, S., Beecher, D., Wong, A., and Ghelardi, A. “Swarming motility in Bacillus cereus and characterization of a fliY mutant impaired in swarm cell differentiation.” Microbiology. 2002. Volume 148. p. 1785-1794.
[15] Pol, I., van Arendonk, W., Mastwijk, H., Krommer, J., Smid, E., and Moezelaar R. “Sensitivities of Germinating Spores and Carvacrol-Adapted Vegetative Cells and Spores of Bacillus cereus to Nisin and Pulsed-Electric-Field Treatment.” Applied Environmental Microbiology. 2001. Volume 67(4). p. 1693–1699.
[16] Kutima, P., and Foegeding, P. “Involvement of the spore coat in germination of Bacillus cereus T spores.” Applied Environmental Microbiology. 1987. Volume 53(1). p.47–52.
[17] Duport, C., Zigha, A., Rosenfeld, E., and Schmitt, P. “Control of Enterotoxin Gene Expression in Bacillus cereus F4430/73 Involves the Redox-Sensitive ResDE Signal Transduction System.” Journal of Bacteriology. 2006. Volume 188. p. 6640–6651.
[18] Mols, M., de Been, M., Zwietering, M., Moezelaar, R., Abee, T. “Metabolic capacity of Bacillus cereus strains ATCC 14579 and ATCC 10987 interlinked with comparative genomics.” Environmental Microbiology. 2007. (Online Early Articles).
[19] Silo-Suh, L., Lethbridge, B., Raffel, S J, He, H., Clardy, J., and Handelsman, J. “Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85.” Applied Environmental Microbiology. 1994. Volume 60(6). p. 2023–2030.
[20] Halverson, L J, and Handelsman J. “Enhancement of soybean nodulation by Bacillus cereus UW85 in the field and in a growth chamber.” Applied Environmental Microbiology. 1991. Volume 57(9). p. 2767–2770.
[21] Peterson, S., Dunn, A., Klimowicz, A., and Handelsman, J. “Peptidoglycan from Bacillus cereus Mediates Commensalism with Rhizosphere Bacteria from the Cytophaga-Flavobacterium Group.” Applied Environmental Microbiology. Volume 72(8). p. 5421–5427.
[22] Margulis, L., Jorgensen, J, Dolan, S., Kolchinsky, R., Rainey, F., and Shyh-Ching. “The Arthromitus stage of Bacillus cereus: Intestinal symbionts of animals.” Proceedings of the National Science Academy U S A. 1998. Volume 3; 95(3). p. 1236–1241.
[23] Jensen, G., Hansen, B., Eilenberg, J., Mahillon, J. “The hidden lifestyles of Bacillus cereus and relatives.” Environmental Microbiology. 2003. Volume 5(8). p. 631–640.
[24] Kotiranta, A., Lounatmaa, K., and Haapasalo, M. “Epidemiology and pathogenesis of Bacillus cereus infections.” Microbes and Infections. 2000. Volume 2, Issue 2. p. 189-198
[25] Granum, P., Lund, T. “Bacillus cereus and its food poisoning toxins.” FEMS Microbiology Letters. 1997. Volume 157 (2). p. 223–228.
[26] Handelsman, J., Jacobson, L., Stabb, E. “Bacillus cereus strain DGA34; United States Patent 5736382.” Official gazette of the United States Patent and Trademark Office. 1998. Accessed August 13, 2007.
[27] Sunaina, V. “Bacterial metabolites from Bacillus cereus B4 responsible for potato plant growth.” Journal of the Indian Potato Association. 2005. Volume 32 (3-4). p. 187-188.
[28] Silo-Suh, L., Lethbridge, B., Raffel, S., He, H., Clardy, J., and Handelsman, J. “Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85.” Applied Environmental Microbiology. 1994. Volume 60(6) p. 2023–2030.
[29] Wijman, J., Leeuw, P., Moezelaar, R., Zwietering, M., and Abee, T. “Air-Liquid Interface Biofilms of Bacillus cereus: Formation, Sporulation, and Dispersion.” Applied Environmental Microbiology. 2007. Volume 73(5). p. 1481–1488.
[30] Clavel, T., Carlin, F., Dargaignaratz, D., Lairon, D., Nguyen-The, C., Schmitt. P. “Effects of porcine bile on survival of Bacillus cereus vegetative cells and Haemolysin BL enterotoxin production in reconstituted human small intestine media.” Journal of Applied Microbiology. 2007(OnlineEarly Articles).
[31] Harvie, D., Vilchez, D., Steggles, J., Ellar, D. “Bacillus cereus Fur regulates iron metabolism and is required for full virulence.” Microbiology. 2005. Volume 151(Pt 2). p.569-77.
Edited by Jacqueline Nguyen student of Rachel Larsen
