Bacillus subtilis: Difference between revisions
Kristal.hess (talk | contribs) |
No edit summary |
||
| (18 intermediate revisions by one other user not shown) | |||
| Line 3: | Line 3: | ||
==Classification== | ==Classification== | ||
===Higher order taxa=== | |||
Domain: Bacteria, phylum: Firmicutes, class: Bacilli, order: Bacillales, family: Bacillaceae | |||
(Entrez Genome Project) | |||
===Genus=== | ===Genus=== | ||
Bacillus | |||
''Bacillus subtilis'' | |||
| Line 21: | Line 21: | ||
|} | |} | ||
==Description and significance== | |||
Originally named ''Vibrio subtilis'' in 1835, this organism was renamed ''Bacillus subtilis'' in 1872. Other names for this bacteria also include ''Bacillus uniflagellatus'', ''Bacillus globigii'', and ''Bacillus natto''. ''Bacillus subtilis'' bacteria were one of the first bacteria to be studied. These bacteria are a good model for cellular development and differentiation (Entrez Genome Project). | |||
''Bacillus subtilis'' cells are rod-shaped, Gram-positive bacteria that are naturally found in soil and vegetation. ''Bacillus subtilis'' grow in the mesophilic temperature range. The optimal temperature is 25-35 degrees Celsius (Entrez Genome Project). Stress and starvation are common in this environment, therefore, ''Bacillus subtilis'' has evolved a set of strategies that allow survival under these harsh conditions. One strategy, for example, is the formation of stress-resistant endospores. | |||
Another strategy is the uptake of external DNA, which allow the bacteria to adapt by recombination. However, these strategies are time-consuming. ''Bacillus subtilis'' can also gain protection more quickly against many stress situations such as acidic, alkaline, osmotic, or oxidative conditions, and heat or ethanol. The alternative sigma factor ?B is a global regulator of stress response. Heat, acid, or ethanol and glucose or phosphate starvation are all stimuli that activate ?B (Bandow 2002). | |||
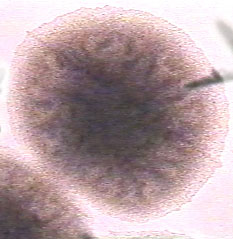
[[Image:Bacillus 1.jpg|frame|center| <i>Bacillus subtilis</i> colony on TSA exhibiting raised, dull, wrinkled characteristics. | |||
<p>image by M. Glogowski with permission]]</p> | |||
==Genome structure== | ==Genome structure== | ||
The genome of Bacillus | Only one DNA molecule is present in these cells. ''Bacillus subtilis'' has one circular chromosome. The total size of all the DNA is 4,214,814 bp (4.2 Mbp) (TIGR CMR). 4,100 genes code for proteins. 53% of the protein-coding genes are only seen once, while 25% of the genome relates to families of genes that have undergone gene duplication (Kunst 1997). | ||
A great portion of the genome corresponds to carbon source applications (Kunst 1997). 192 of the 4,100 genes are considered indispensable, and an additional 79 are thought to be essential. Most of the essential genes are involved in metabolism. Half of the essential genes are responsible for processing information, one-fifth of them are responsible for cell wall synthesis, cell division and shape, and one-tenth of them were responsible for the energetics of the cell. The essential genes that code for functions that are not known are 4% (Kobayashi 2003). ''Bacillus subtilis'' bacteria are capable of secreting antibiotics in great numbers to the exterior of the cell (Ara 2007). Five signal peptidase genes were found to be important for this secretion function. Many of ''Bacillus subtilis'' cells' genes are responsible for antibiotic synthesis (Kunst 1997). | |||
==Cell structure and metabolism== | |||
''Bacillus subtilis'' are rod-shaped bacteria that are Gram-positive (Perez 2000). The cell wall is a rigid structure outside the cell. It is composed of peptidoglycan, which is a polymer of sugars and amino acids. The peptidoglycan that is found in bacteria is known as murein. Other constituents that extend from the murein are teichoic acids, lipoteichoic acids, and proteins. The cell wall forms the barrier between the environment and the bacterial cell. It is also responsible for maintaining the shape of the cell and withstanding the cell's high internal turgor pressure (Schaechter 2006). | |||
<i>B. subtilis</i> is capable of butanediol fermentation. It does not hydrolyze phospholipids nor casein; it does hydrolyze triglycerides. It produces citrate permease and cytochrome c. | |||
''Bacillus subtilis'' is a model organism for studying endospore formation in bacteria. Endospores in ''Bacillus subtilis'' bacteria are mostly formed in the tips of protuberances extending downward from liquid surface pellicles (Schaechter 2006). Many strains produce spores with brown pigments. Depletion of carbon, nitrogen, or phosphorous causes the process of sporulation to begin, however, the process needs to start before the entire exhaustion of nutrients (Perez 2000). Otherwise, the spore formation cannot be completed due to the fact that the nutrients are too low for the energy-requiring sporulation process. This allows the cells to avoid being stuck in a vulnerable position. | |||
The formation of the endospore occurs in several stages, denoted 0 through VI. Sporulation occurs in the following fashion. First the nucleoid lengthens, becoming an axial filament. Then, the cell forms a polar septum, one-fourth of the cell length from one end, and begins to divide. The smaller product of this division is called the forespore and the larger product is called the mother cell (Perez 2000). The mother cell is responsible for nourishing the newly formed spore. When the septum forms, 30% of the chromosome is already on the forespore side (Schaechter 2006). The remaining 70% of the chromosome enters the forespore in a fashion similar to DNA transfer during conjugation; it is pumped by a protein called SpoIIIE. The mother cell then engulfs the forespore by acting like a phagocyte. This causes the forespore to have two cytoplasmic membranes with a thick murein layer, namely the cortex, between them. A protein spore coat and an exosporium, a membranous layer, form outside of the forespore membranes. At this time, the forespore undergoes internal changes. Lastly, the forespore leaves the mother cell upon lysis of the mother cell (Perez 2000). A mature endospore has no metabolic activity; it is inert. The interior of the endospore, the core, is very dry and resistant to moisture (Schaechter 2006). | |||
[[Image:B_subtilis_Spore_2.jpg|frame|center| <i>Bacillus subtilis</i> subterminal endospore. | |||
<p>image by M. Glogowski with permission]]</p> | |||
''Bacillus subtilis'' bacteria use their flagella for a swarming motility. This motility occurs on surfaces, for example on agar plates, rather than in liquids. ''Bacillus subtilis'' are arranged in singles or chains. Cells arranged next to each other can only swarm together, not individually. These arrangements of cells are called 'rafts'. In order for ''Bacillus subtilis'' bacteria to swarm, they need to secrete a slime layer which includes surfactin, a surface tension-reducing lipopeptide, as one of its components (Schaechter 2006). | |||
Bacillus | |||
''Bacillus subtilis'' bacteria have been considered strictly aerobic, meaning that they require oxygen to grow and they cannot undergo fermentation. However, recent studies show that they can indeed grow in anaerobic conditions making them facultative aerobes. The bacteria can make ATP in anaerobic conditions via butanediol fermentation as well as nitrate ammonification. ''Bacillus subtilis'' can use nitrite or nitrate as a terminal acceptor of electrons. ''Bacillus subtilis'' contains two unique nitrate reductases. One is used for nitrate nitrogen assimilation and the other is used for nitrate respiration. However, there is only one nitrite reductase that serves both purposes. Nitrate reductase reduces nitrate to nitrite in nitrate respiration, which is then reduced to ammonia by nitrite reductase. ''Bacillus subtilis'' is different from other facultative aerobes in that it undergoes fermentation without external acceptors of electrons (Nakano 1998). During fermentation, the regeneration of NAD+ is chiefly mediated by lactate dehydrogenase, which is found in the cytoplasm. Lactate dehydrogenase converts pyruvate to lactate (Marino 2001). | |||
''Bacillus subtilis'' contains catalase KatA and MrgA, an enzyme that is responsible in the catalysis of the decomposition of hydrogen peroxide to water and oxygen, and superoxide dismutase, an enzyme that catalyzes the breakdown of superoxide into oxygen and hydrogen peroxide (Bandow 2002). | |||
==Chromosome Replication== | |||
''Bacillus subtilis'' duplicates its single circular chromosome by initiating DNA replication at a single locus, the origin (''oriC''). Replication proceeds bidirectionally and two replication forks progress in the clockwise and counterclockwise directions along the chromosome halves. Chromosome replication is completed when the forks reach the terminus region, which is positioned opposite to the origin on the chromosome map, and contains several short DNA sequences (''Ter'' sites) that promote replication arrest. Specific proteins mediate all the steps in DNA replication. The comparison between the sets of proteins involved in chromosomal DNA replication in ''B. subtilis'' and in ''Escherichia coli'' reveals both similarities and differences. Although the basic components promoting initiation, elongation, and termination of replication are well conserved, some important differences can be found (such as one bacterium missing proteins essential in the other). These differences underline the diversity in the mechanisms and strategies that various bacterial species have adopted to carry out the duplication of their genomes (Graumann, 2007). | |||
==Ecology== | ==Ecology== | ||
The main habitat of endospore forming ''Bacillus'' organisms is the soil. Likewise <i>Bacillus subtilis</i> is most commonly found in soil environments and on plant undergrowth. These mesophilic microbes have historically been considered strict aerobes. Thus they are likely to be found in O and A surface soil horizons where the concentration of oxygen is most abundant and temperatures are relatively mild. Consider how this organism functions in s competitive microbial community: when carbon-, nitrogen- and phosphorus-nutrient levels fall below the bacterium's optimal threshold, it produces spores. Scientists have demonstrated that <i>Bacillus subtilis</i> concurrently produces antibiotics and spores. Antibiotic production increases B. Subtilis's cance at survival as the organism produces spores and a toxin that might kill surrounding gram positive microbes that compete for the same nutrients. | |||
These microbes form spores in times of nutrient exhaustion. When the nutrients required for the bacteria to grow are abundant, they exhibit metabolic activity. These organisms can produce antibiotics during sporulation. Examples of the antibiotics that ''Bacillus subtilis'' can produce include are polymyxin, difficidin, subtilin, and mycobacillin. Many of the ''Bacillus'' microbes can degrade polymers such as protein, starch, and pectin, therefore, they are thought to be an important contributor to the carbon and nitrogen cycles. When they cause contamination, they may result in decomposition. Quite a few of the ''Bacillus'' organisms are primarily responsible for the spoilage of food (Todar). | |||
<i>Bacillus subtilis</i> supports plant browth. As a member of <i>Bacillus</i>, this bacterium often plays a role in replenishing soil nutrients by supplying the terrestrial carbon cycle and the nitrogen cycle. ''Bacillus subtilis'' bacteria form rough biofilms, which are dense organism communities, at the air and water interface. ''Bacillus subtilis'' biofilms are beneficial. They allow for the control of plant pathogen infections. <i>B. subtilis</i> biofilm communities form a mutualistic interaction with plant rhizome systems. The plant benefits because <i>B. subtilis</i> provides preemptive colonization. Preemptive colonization prevents other pathogens from infecting the plant because <i>B. subtilis</i> has the advantage of being at the site first. The biofilm communities form a mutualistic interaction with plant rhizome systems. ''Bacillus subtilis'' biofilms found in the rhizosphere of plants promote growth and serve as a biocontroller. In this sense, <i>B. subtilis</i> biofilm communities form a mutualistic interaction with plant rhizome systems. The plant benefits because <i>B. subtilis</i> provides preemptive colonizatiion. B. subtilis benefits by deriving nutrients and surface area for biofilm formatiion from the plant's root structure. ''Bacillus subtilis'' strains can act as biofungicides for benefiting agricultural crops and antibacterial agents. ''Bacillus subtilis'' also reduces mild steel corrosion (Morikawa 2006). | |||
==Pathology== | ==Pathology== | ||
| Line 62: | Line 85: | ||
Some ''Bacillus'' organisms can cause more severe illnesses. ''[[Bacillus anthracis]]'', for example, causes Anthrax. It was the first bacterial organism that was known to cause disease in humans. ''[[Bacillus anthracis]]'' spores can survive for very long periods of time. Anthrax is very rare in humans, however it is more common in animals. The disease often begins with a very high fever and chest pain, and can be fatal if untreated (EMBL EBI). | Some ''Bacillus'' organisms can cause more severe illnesses. ''[[Bacillus anthracis]]'', for example, causes Anthrax. It was the first bacterial organism that was known to cause disease in humans. ''[[Bacillus anthracis]]'' spores can survive for very long periods of time. Anthrax is very rare in humans, however it is more common in animals. The disease often begins with a very high fever and chest pain, and can be fatal if untreated (EMBL EBI). | ||
==Application to Biotechnology== | |||
''Bacillus'' organisms, isolated by soil sprinkle technique, are responsible for producing antibiotics. The most antibiotic activity was seen in ''Bacillus subtilis'' MH-4. The most optimal activity occurs at a temperature of 37 degrees Celsius and a basic pH of 8. Glycerol is the optimal carbon source and L-glutamic acid is the optimal source of nitrogen. The antibiotic bacitracin was determined to be affective on Gram-positive bacteria only (Jamil 2007). Other antibiotics that ''Bacillus subtilis'' form are polymyxin, difficidin, subtilin, and mycobacillin. Polymyxin is affective against Gram-negative bacteria, whereas difficidin has a broader spectrum (Todar). | |||
''Bacillus subtilis'' bacteria secrete enzymes, "such as amylase, protease, pullulanase, chitinase, xylanase, lipase, among others. These enzymes are produced commercially and this enzyme production represents about 60% of the commercially produced industrial enzymes" (Morikawa 2006). | |||
==Current Research== | ==Current Research== | ||
There are many research studies that are currently being done on ''Bacillus subtilis''. One recent research project focuses on the resistance of ''Bacillus subtilis'' spores to heat, radiation, and chemicals. It has been known that spores can survive hundreds, even millions, of years in a dormant state. The study investigated the important factors that contribute to spore resistance. The researcher found that the bacteria's coats were a major factor because the coat provides a barrier for the organism against toxic agents, ultraviolet radiation, and lytic enzymes. The inner membrane was also found to be important, due to its low permeability against toxic agents. DNA repair was also determined to be crucial, since it can control DNA damage due to radiation, heat, and toxins. ''Bacillus subtilis'' spores are also resistant to wet heat, primarily by the core's low water content. The lower the water content of the core is, the more resistant the spore is to wet heat. This research study is important in that it can lead to future studies on how the ''Bacillus subtilis'' spores in food and medical products can be killed effectively. Learning about the spores resistance gives us a better understanding of which methods may or may not be useful in killing the spores (Setlow 2006). | |||
Another current research study provides evidence that the SpoIIIE DNA translocase is required for ''Bacillus subtilis'' forespore chromosome translocation across the septum and membrane fusion during sporulation. The researchers studied SpoIIIE mutants. They found that one mutant undergoes the translocation of DNA, but does not undergo membrane fusion normally after the engulfment. They discovered that the septum stays open in this mutation. When the sporulation septum is open, the cytoplasm is permitted to be exchanged between the daughter cells. This implies that the membrane does not fuse properly after engulfment and cytokinesis. The researchers proposed "that SpoIIIE catalyses these topologically opposite fusion events by assembling or disassembling a proteinaceous fusion pore" (Liu 2006). The study demonstrated that SpoIIIE first participates in allowing a barrier of diffusion for the translocation of DNA, and then participates in the fusion of the membrane. Thus, SpoIIIE is required for the fusion of the engulfing membrane after the engulfment of the forespore (Liu 2006). | |||
A third current research project investigates ''Bacillus subtilis'' fermented soybean meal and its effects on enzymes in the gastrointestinal tract and intestinal morphology of piglets. The piglets were randomly given either soybean meal or fermented soybean meal. After the experiment was completed, the six piglets from each of the two treatment groups were sacrificed. The contents of the small intestine were collected, and the tissue was sampled at varying locations. The researchers found, using light microscopy, that the piglets in the treatment group that were fed fermented soybean meal had significantly taller villi at the varying locations, and had a significantly lower duodenal crypt depth in comparison to the piglets in the treatment group that were fed soybean meal. They also showed a significant increase in duodenal and jejunal protease and trypsin activities and a decrease in pancreatic trypsin activity. The findings obtained from this research demonstrate that fermented soybean meal improves the morphology of the intestine as well as the activities of digestive enzymes (Feng 2007). | |||
==References== | ==References== | ||
Latest revision as of 14:31, 7 June 2015
A Microbial Biorealm page on the genus Bacillus subtilis
Classification
Higher order taxa
Domain: Bacteria, phylum: Firmicutes, class: Bacilli, order: Bacillales, family: Bacillaceae (Entrez Genome Project)
Genus
Bacillus subtilis
|
NCBI: Taxonomy |
Description and significance
Originally named Vibrio subtilis in 1835, this organism was renamed Bacillus subtilis in 1872. Other names for this bacteria also include Bacillus uniflagellatus, Bacillus globigii, and Bacillus natto. Bacillus subtilis bacteria were one of the first bacteria to be studied. These bacteria are a good model for cellular development and differentiation (Entrez Genome Project).
Bacillus subtilis cells are rod-shaped, Gram-positive bacteria that are naturally found in soil and vegetation. Bacillus subtilis grow in the mesophilic temperature range. The optimal temperature is 25-35 degrees Celsius (Entrez Genome Project). Stress and starvation are common in this environment, therefore, Bacillus subtilis has evolved a set of strategies that allow survival under these harsh conditions. One strategy, for example, is the formation of stress-resistant endospores.
Another strategy is the uptake of external DNA, which allow the bacteria to adapt by recombination. However, these strategies are time-consuming. Bacillus subtilis can also gain protection more quickly against many stress situations such as acidic, alkaline, osmotic, or oxidative conditions, and heat or ethanol. The alternative sigma factor ?B is a global regulator of stress response. Heat, acid, or ethanol and glucose or phosphate starvation are all stimuli that activate ?B (Bandow 2002).
Genome structure
Only one DNA molecule is present in these cells. Bacillus subtilis has one circular chromosome. The total size of all the DNA is 4,214,814 bp (4.2 Mbp) (TIGR CMR). 4,100 genes code for proteins. 53% of the protein-coding genes are only seen once, while 25% of the genome relates to families of genes that have undergone gene duplication (Kunst 1997).
A great portion of the genome corresponds to carbon source applications (Kunst 1997). 192 of the 4,100 genes are considered indispensable, and an additional 79 are thought to be essential. Most of the essential genes are involved in metabolism. Half of the essential genes are responsible for processing information, one-fifth of them are responsible for cell wall synthesis, cell division and shape, and one-tenth of them were responsible for the energetics of the cell. The essential genes that code for functions that are not known are 4% (Kobayashi 2003). Bacillus subtilis bacteria are capable of secreting antibiotics in great numbers to the exterior of the cell (Ara 2007). Five signal peptidase genes were found to be important for this secretion function. Many of Bacillus subtilis cells' genes are responsible for antibiotic synthesis (Kunst 1997).
Cell structure and metabolism
Bacillus subtilis are rod-shaped bacteria that are Gram-positive (Perez 2000). The cell wall is a rigid structure outside the cell. It is composed of peptidoglycan, which is a polymer of sugars and amino acids. The peptidoglycan that is found in bacteria is known as murein. Other constituents that extend from the murein are teichoic acids, lipoteichoic acids, and proteins. The cell wall forms the barrier between the environment and the bacterial cell. It is also responsible for maintaining the shape of the cell and withstanding the cell's high internal turgor pressure (Schaechter 2006).
B. subtilis is capable of butanediol fermentation. It does not hydrolyze phospholipids nor casein; it does hydrolyze triglycerides. It produces citrate permease and cytochrome c.
Bacillus subtilis is a model organism for studying endospore formation in bacteria. Endospores in Bacillus subtilis bacteria are mostly formed in the tips of protuberances extending downward from liquid surface pellicles (Schaechter 2006). Many strains produce spores with brown pigments. Depletion of carbon, nitrogen, or phosphorous causes the process of sporulation to begin, however, the process needs to start before the entire exhaustion of nutrients (Perez 2000). Otherwise, the spore formation cannot be completed due to the fact that the nutrients are too low for the energy-requiring sporulation process. This allows the cells to avoid being stuck in a vulnerable position.
The formation of the endospore occurs in several stages, denoted 0 through VI. Sporulation occurs in the following fashion. First the nucleoid lengthens, becoming an axial filament. Then, the cell forms a polar septum, one-fourth of the cell length from one end, and begins to divide. The smaller product of this division is called the forespore and the larger product is called the mother cell (Perez 2000). The mother cell is responsible for nourishing the newly formed spore. When the septum forms, 30% of the chromosome is already on the forespore side (Schaechter 2006). The remaining 70% of the chromosome enters the forespore in a fashion similar to DNA transfer during conjugation; it is pumped by a protein called SpoIIIE. The mother cell then engulfs the forespore by acting like a phagocyte. This causes the forespore to have two cytoplasmic membranes with a thick murein layer, namely the cortex, between them. A protein spore coat and an exosporium, a membranous layer, form outside of the forespore membranes. At this time, the forespore undergoes internal changes. Lastly, the forespore leaves the mother cell upon lysis of the mother cell (Perez 2000). A mature endospore has no metabolic activity; it is inert. The interior of the endospore, the core, is very dry and resistant to moisture (Schaechter 2006).
Bacillus subtilis bacteria use their flagella for a swarming motility. This motility occurs on surfaces, for example on agar plates, rather than in liquids. Bacillus subtilis are arranged in singles or chains. Cells arranged next to each other can only swarm together, not individually. These arrangements of cells are called 'rafts'. In order for Bacillus subtilis bacteria to swarm, they need to secrete a slime layer which includes surfactin, a surface tension-reducing lipopeptide, as one of its components (Schaechter 2006).
Bacillus subtilis bacteria have been considered strictly aerobic, meaning that they require oxygen to grow and they cannot undergo fermentation. However, recent studies show that they can indeed grow in anaerobic conditions making them facultative aerobes. The bacteria can make ATP in anaerobic conditions via butanediol fermentation as well as nitrate ammonification. Bacillus subtilis can use nitrite or nitrate as a terminal acceptor of electrons. Bacillus subtilis contains two unique nitrate reductases. One is used for nitrate nitrogen assimilation and the other is used for nitrate respiration. However, there is only one nitrite reductase that serves both purposes. Nitrate reductase reduces nitrate to nitrite in nitrate respiration, which is then reduced to ammonia by nitrite reductase. Bacillus subtilis is different from other facultative aerobes in that it undergoes fermentation without external acceptors of electrons (Nakano 1998). During fermentation, the regeneration of NAD+ is chiefly mediated by lactate dehydrogenase, which is found in the cytoplasm. Lactate dehydrogenase converts pyruvate to lactate (Marino 2001).
Bacillus subtilis contains catalase KatA and MrgA, an enzyme that is responsible in the catalysis of the decomposition of hydrogen peroxide to water and oxygen, and superoxide dismutase, an enzyme that catalyzes the breakdown of superoxide into oxygen and hydrogen peroxide (Bandow 2002).
Chromosome Replication
Bacillus subtilis duplicates its single circular chromosome by initiating DNA replication at a single locus, the origin (oriC). Replication proceeds bidirectionally and two replication forks progress in the clockwise and counterclockwise directions along the chromosome halves. Chromosome replication is completed when the forks reach the terminus region, which is positioned opposite to the origin on the chromosome map, and contains several short DNA sequences (Ter sites) that promote replication arrest. Specific proteins mediate all the steps in DNA replication. The comparison between the sets of proteins involved in chromosomal DNA replication in B. subtilis and in Escherichia coli reveals both similarities and differences. Although the basic components promoting initiation, elongation, and termination of replication are well conserved, some important differences can be found (such as one bacterium missing proteins essential in the other). These differences underline the diversity in the mechanisms and strategies that various bacterial species have adopted to carry out the duplication of their genomes (Graumann, 2007).
Ecology
The main habitat of endospore forming Bacillus organisms is the soil. Likewise Bacillus subtilis is most commonly found in soil environments and on plant undergrowth. These mesophilic microbes have historically been considered strict aerobes. Thus they are likely to be found in O and A surface soil horizons where the concentration of oxygen is most abundant and temperatures are relatively mild. Consider how this organism functions in s competitive microbial community: when carbon-, nitrogen- and phosphorus-nutrient levels fall below the bacterium's optimal threshold, it produces spores. Scientists have demonstrated that Bacillus subtilis concurrently produces antibiotics and spores. Antibiotic production increases B. Subtilis's cance at survival as the organism produces spores and a toxin that might kill surrounding gram positive microbes that compete for the same nutrients.
These microbes form spores in times of nutrient exhaustion. When the nutrients required for the bacteria to grow are abundant, they exhibit metabolic activity. These organisms can produce antibiotics during sporulation. Examples of the antibiotics that Bacillus subtilis can produce include are polymyxin, difficidin, subtilin, and mycobacillin. Many of the Bacillus microbes can degrade polymers such as protein, starch, and pectin, therefore, they are thought to be an important contributor to the carbon and nitrogen cycles. When they cause contamination, they may result in decomposition. Quite a few of the Bacillus organisms are primarily responsible for the spoilage of food (Todar).
Bacillus subtilis supports plant browth. As a member of Bacillus, this bacterium often plays a role in replenishing soil nutrients by supplying the terrestrial carbon cycle and the nitrogen cycle. Bacillus subtilis bacteria form rough biofilms, which are dense organism communities, at the air and water interface. Bacillus subtilis biofilms are beneficial. They allow for the control of plant pathogen infections. B. subtilis biofilm communities form a mutualistic interaction with plant rhizome systems. The plant benefits because B. subtilis provides preemptive colonization. Preemptive colonization prevents other pathogens from infecting the plant because B. subtilis has the advantage of being at the site first. The biofilm communities form a mutualistic interaction with plant rhizome systems. Bacillus subtilis biofilms found in the rhizosphere of plants promote growth and serve as a biocontroller. In this sense, B. subtilis biofilm communities form a mutualistic interaction with plant rhizome systems. The plant benefits because B. subtilis provides preemptive colonizatiion. B. subtilis benefits by deriving nutrients and surface area for biofilm formatiion from the plant's root structure. Bacillus subtilis strains can act as biofungicides for benefiting agricultural crops and antibacterial agents. Bacillus subtilis also reduces mild steel corrosion (Morikawa 2006).
Pathology
Bacillus subtilis bacteria are non-pathogenic. They can contaminate food, however, they seldom result in food poisoning. They are used on plants as a fungicide. They are also used on agricultural seeds, such as vegetable and soybean seeds, as a fungicide. The bacteria, colonized on root systems, compete with disease causing fungal organisms. Bacillus subtilis use as a fungicide fortunately does not affect humans (EMBL EBI). Some strains of Bacillus subtilis cause rots in potatoes. It grows in food that is non-acidic, and can cause ropiness in bread that is spoiled (Todar). Some strains related to Bacillus subtilis are capable of producing toxins for insects. Those strains can also be used for protecting crops as well. Bacillus thuringiensis, for example, is another bacterium in the same genus that is used for insect control (EMBL EBI).
Some Bacillus species can cause food poisoning, such as Bacillus cereus and Bacillus licheniformis. Bacillus cereus can result in two different kinds of intoxications. It can either cause nausea, vomiting, and abdominal cramps for 1-6 hours, or diarrhea and abdominal cramps for 8-16 hours. The food poisoning usually occurs from eating rice that is contaminated with Bacillus cereus (EMBL EBI).
Some Bacillus organisms can cause more severe illnesses. Bacillus anthracis, for example, causes Anthrax. It was the first bacterial organism that was known to cause disease in humans. Bacillus anthracis spores can survive for very long periods of time. Anthrax is very rare in humans, however it is more common in animals. The disease often begins with a very high fever and chest pain, and can be fatal if untreated (EMBL EBI).
Application to Biotechnology
Bacillus organisms, isolated by soil sprinkle technique, are responsible for producing antibiotics. The most antibiotic activity was seen in Bacillus subtilis MH-4. The most optimal activity occurs at a temperature of 37 degrees Celsius and a basic pH of 8. Glycerol is the optimal carbon source and L-glutamic acid is the optimal source of nitrogen. The antibiotic bacitracin was determined to be affective on Gram-positive bacteria only (Jamil 2007). Other antibiotics that Bacillus subtilis form are polymyxin, difficidin, subtilin, and mycobacillin. Polymyxin is affective against Gram-negative bacteria, whereas difficidin has a broader spectrum (Todar).
Bacillus subtilis bacteria secrete enzymes, "such as amylase, protease, pullulanase, chitinase, xylanase, lipase, among others. These enzymes are produced commercially and this enzyme production represents about 60% of the commercially produced industrial enzymes" (Morikawa 2006).
Current Research
There are many research studies that are currently being done on Bacillus subtilis. One recent research project focuses on the resistance of Bacillus subtilis spores to heat, radiation, and chemicals. It has been known that spores can survive hundreds, even millions, of years in a dormant state. The study investigated the important factors that contribute to spore resistance. The researcher found that the bacteria's coats were a major factor because the coat provides a barrier for the organism against toxic agents, ultraviolet radiation, and lytic enzymes. The inner membrane was also found to be important, due to its low permeability against toxic agents. DNA repair was also determined to be crucial, since it can control DNA damage due to radiation, heat, and toxins. Bacillus subtilis spores are also resistant to wet heat, primarily by the core's low water content. The lower the water content of the core is, the more resistant the spore is to wet heat. This research study is important in that it can lead to future studies on how the Bacillus subtilis spores in food and medical products can be killed effectively. Learning about the spores resistance gives us a better understanding of which methods may or may not be useful in killing the spores (Setlow 2006).
Another current research study provides evidence that the SpoIIIE DNA translocase is required for Bacillus subtilis forespore chromosome translocation across the septum and membrane fusion during sporulation. The researchers studied SpoIIIE mutants. They found that one mutant undergoes the translocation of DNA, but does not undergo membrane fusion normally after the engulfment. They discovered that the septum stays open in this mutation. When the sporulation septum is open, the cytoplasm is permitted to be exchanged between the daughter cells. This implies that the membrane does not fuse properly after engulfment and cytokinesis. The researchers proposed "that SpoIIIE catalyses these topologically opposite fusion events by assembling or disassembling a proteinaceous fusion pore" (Liu 2006). The study demonstrated that SpoIIIE first participates in allowing a barrier of diffusion for the translocation of DNA, and then participates in the fusion of the membrane. Thus, SpoIIIE is required for the fusion of the engulfing membrane after the engulfment of the forespore (Liu 2006).
A third current research project investigates Bacillus subtilis fermented soybean meal and its effects on enzymes in the gastrointestinal tract and intestinal morphology of piglets. The piglets were randomly given either soybean meal or fermented soybean meal. After the experiment was completed, the six piglets from each of the two treatment groups were sacrificed. The contents of the small intestine were collected, and the tissue was sampled at varying locations. The researchers found, using light microscopy, that the piglets in the treatment group that were fed fermented soybean meal had significantly taller villi at the varying locations, and had a significantly lower duodenal crypt depth in comparison to the piglets in the treatment group that were fed soybean meal. They also showed a significant increase in duodenal and jejunal protease and trypsin activities and a decrease in pancreatic trypsin activity. The findings obtained from this research demonstrate that fermented soybean meal improves the morphology of the intestine as well as the activities of digestive enzymes (Feng 2007).
References
[1] Ara, K., et al. "Bacillus minimum genome factory: effective utilization of microbial genome information." Biotechnol. Appl. Biochem.. 2007 March; 46(Pt 3):169-78.
[2] Bandow, J.E., H. Br�tz, M. Hecker. "Bacillus subtilis Tolerance of Moderate Concentrations of Rifampin Involves the ?B-Dependent General and Multiple Stress Response". Journal of Bacteriology. 2002 January; 184(2): 459�467.
[3]
Entrez Genome Project, NCBI
[4]
European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL EBI).
[5]
Feng, J., et al. "Effect of Fermented Soybean Meal on Intestinal Morphology and Digestive Enzyme Activities in Weaned Piglets". Digestive Diseases and Sciences. 2007 April.
[6]
Graumann, P. 2007. Bacillus: Cellular and Molecular Biology. Caister Academic press. ISBN 978-1-904455-12-7.
[7]
Jamil, B., et al. "Isolation of Bacillus subtilis MH-4 from Soil and its Potential of Polypeptidic Antibiotic Production". Pak J Pharm Sci. 2007 January; 20(1):26-31.
[8]
Kobayashi, K., et al. "Essential Bacillus subtilis genes". Proc Natl Acad Sci U S A. 2003 April 15; 100(8): 4678�4683.
[9]
Kunst, F., et al. "The complete genome sequence of the Gram-positive bacterium Bacillus subtilis". Nature. 1997 November; 390, 249-256.
[10]
Liu, NJ., RJ. Dutton, K. Pogliano. "Evidence that the SpoIIIE DNA Translocase Participates in Membrane Fusion During Cytokinesis and Engulfment". Mol Microbiol 2006 February;59(4):1097-113.
[11]
Marino, M., et al. "Modulation of Anaerobic Energy Metabolism of Bacillus subtilis by arfM (ywiD)". J Bacteriol. 2001 December; 183(23): 6815�6821.
[12]
Morikawa, M. "Beneficial Biofilm Formation by Industrial Bacteria Bacillus subtilis and Related Species". Journal of Bioscience and Bioengineering. 2006; Vol.101, No.1, 1-8.
[13] Nakano, M.M., P. Zuber. "Anaerobic Growth of a 'Strict Aerobe' (Bacillus subtilis)". Annual Review of Microbiology. 1998 October; Vol. 52: 165-190.
[14] Perez, A.R., A. Abanes-De Mello, K. Pogliano. "SpoIIB Localizes to Active Sites of Septal Biogenesis and Spatially Regulates Septal Thinning during Engulfment in Bacillus subtilis". Journal of Bacteriology. 2000 February; 182(4): 1096�1108.
Schaechter, M., J.L. Ingraham, F.C. Neidhardt. Microbe. (ASM Press, Washington, DC, 2006).
[15]
Setlow, P. "Spores of Bacillus subtilis:Their Resistance to and Killing by Radiation, Heat, and Chemicals". Journal of Applied Microbiology. 2006 September; 101(3), 514-525.
[16]
The Institute for Genome Research, Comprehensive Microbial Resource (TIGR CMR).
[17]
Todar, K. "Todar's Online Textbook of Bacteriology".
Edited by Margo Ucar, student of Rachel Larsen and Kit Pogliano
Edited by a student of M Glogowski at Loyola University