Bacterial phage defense mechanisms with applications: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
==Restriction Modification== | ==Restriction Modification== | ||
Restriction modification (R-M) is a defense mechanism which is widely spread among | Restriction modification (R-M) is a defense mechanism which is widely spread among bacteria [[#References|[3]]]. There are several types of R-M and all of these typically involve at least two enzymes, a restriction [http://en.wikipedia.org/wiki/Endonuclease endonuclease] (REase) and a [http://en.wikipedia.org/wiki/Methyltransferase methyltransferase] (MTase). The REase is responsible for the cleavage of intruding double-stranded DNA, e.g. phage genomes4, through the recognition of the specific nucleotide restriction sites. Upon genome cleavage the phage is not able to finish its life cycle. [http://en.wikipedia.org/wiki/Methylation Methylation] of restriction sites by MTase protects the host cell genome from cleavage and REases are categorized as different restriction endonuclease types depending on their specific mode of activity [[#References|[3]]]. | ||
There are three officially recognized ''Ignicoccus'' species: ''Ignicoccus hospitalis'' , '' Ignicoccus pacificus '' and '' Ignicoccus islandicus'' . The three species were initially identified by 16S rRNA gene analysis from the hydrothermal vent samples obtained from Kolbeinsey Ridge and the coast of Mexico[[#References|[1]]] . All three species have been characterized as hyperthermophiles that are also [http://en.wikipedia.org/wiki/Obligate_anaerobe obligate anaerobes] which explains the presence of ''Ignicoccus'' species near hydrothermal vents[[#References|[1]]] . None of the members of the ''Ignicoccus'' genus have been found to be [http://en.wikipedia.org/wiki/Pathogenic] pathogenic to humans. | There are three officially recognized ''Ignicoccus'' species: ''Ignicoccus hospitalis'' , '' Ignicoccus pacificus '' and '' Ignicoccus islandicus'' . The three species were initially identified by 16S rRNA gene analysis from the hydrothermal vent samples obtained from Kolbeinsey Ridge and the coast of Mexico[[#References|[1]]] . All three species have been characterized as hyperthermophiles that are also [http://en.wikipedia.org/wiki/Obligate_anaerobe obligate anaerobes] which explains the presence of ''Ignicoccus'' species near hydrothermal vents[[#References|[1]]] . None of the members of the ''Ignicoccus'' genus have been found to be [http://en.wikipedia.org/wiki/Pathogenic] pathogenic to humans. | ||
Revision as of 02:03, 30 November 2013
Overview
Bacteria are constantly subjected to bacteriophages and other selfish genetic elements. Bacteriophages are viruses that specifically infect bacteria and the relationship can be described as a parasitic. This is because bacteria are harmed throughout the phage replication cycle and often lysed when progeny phage particles leave the cell [1]. Moreover, it is estimated that there are 10^30-10^32 total phage particles on earth, which outnumber bacteria by 10-fold [2]. This means that phages are found in almost every environment in which bacteria exist, making virtually all bacteria susceptible to phage infection. In response to this constant exposure to phage, bacteria have evolved several diverse antiviral defense mechanisms. These mechanisms include adsorption blocking, uptake block, abortive infection, restriction modification and the CRISPR-Cas system [1]. Restriction modification and the CRISPR-Cas system are elaborated on in this article as they are the best understood and important tools in molecular biology.
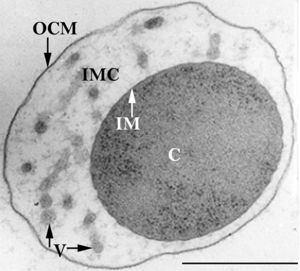
To date, the genus Ignicoccus is comprised of single cells that are irregularly shaped coccoid ranging in diameter from 1-3 µm. The Achaean genus was first isolated from marine hydrothermal vents from Kolbeinsey Ridge in north Iceland and also off the coast of Mexico (see Figure 1). They were found to have a novel cell envelope unseen before in other Achaea ], and have a very complex and poorly understood symbiotic relationship with Nanoarchaeum equitans [3] [4] [5] [6].
Key Defense Mechanisms
Restriction Modification
Restriction modification (R-M) is a defense mechanism which is widely spread among bacteria [3]. There are several types of R-M and all of these typically involve at least two enzymes, a restriction endonuclease (REase) and a methyltransferase (MTase). The REase is responsible for the cleavage of intruding double-stranded DNA, e.g. phage genomes4, through the recognition of the specific nucleotide restriction sites. Upon genome cleavage the phage is not able to finish its life cycle. Methylation of restriction sites by MTase protects the host cell genome from cleavage and REases are categorized as different restriction endonuclease types depending on their specific mode of activity [3].
There are three officially recognized Ignicoccus species: Ignicoccus hospitalis , Ignicoccus pacificus and Ignicoccus islandicus . The three species were initially identified by 16S rRNA gene analysis from the hydrothermal vent samples obtained from Kolbeinsey Ridge and the coast of Mexico[1] . All three species have been characterized as hyperthermophiles that are also obligate anaerobes which explains the presence of Ignicoccus species near hydrothermal vents[1] . None of the members of the Ignicoccus genus have been found to be [1] pathogenic to humans.
CRISPR
The CRISPR-Cas system is a mechanism that evolved in bacteria and archea to protect against genetic element intrusion and functions similarly to an adaptive immune system. Clustered regularly interspaced short palindromic repeats (CRISPR), are loci with several non-continuous direct repeats separated by stretches of variable sequences called spacers7 . These repeat and spacer sequences, along with one or several cas (CRISPR associated) genes, are key elements present in every CRISPR-Cas system mechanism7.
There are three known types of CRISPR-Cas systems as well as several diverse subtypes. The most commonly used CRISPR-Cas system in molecular biology is type II, which is naturally found in Streptococcus thermophiles, a lactic acid bacterium that is important to the dairy industry. Cas 9 is the key enzyme required for the CRISPR system to function and has several enzymatic functions, including endonuclease and integrase activities7,8 . Cas 9 recognizes specific dsDNA sequences in the phage genome called protospacer adjacent motive (PAM), and uptakes a prospacer nucleotide sequence of about 30 bases downstream of the PAM site. This sequence is then integrated by Cas9 as a spacer into the bacterial genome flanked by two repeat sequences. The spacer sequence then gets transcribed into a precursor CRISPR RNA (pre-crRNA). The pre-crRNA gets processed by an RNase which is triggered through a transactivating crRNA (tracrRNA) that is complementary to the repeat. The process happens in the presence of Cas 9, which then associates with the processed crRNA 8. Upon re-encounter of the same phage genome, site-specific cleavage by Cas 9 occurs as the target site is determined by base complementarity between crRNA and the prospacer in the phage genome 8.
The members of the Ignicoccus genus are motile irregular coccoid cells that range in diameter from 1 to 3 µm. The motility observed is due to the presence of flagella, but unfortunately the polarity of the flagella is not yet fully elucidated. They are known to have an outer-membrane but no S-layer. This is a novel characteristic for these Archaea becauseIgnicoccus are the only known Archaea that have been shown to possess an outer-membrane[2] [10] .
Bacteria and Phage Arms Race
Bacteria and phage are in a constant arm race of co-evolving defense mechanisms. For example, while bacterial defense mechanisms like CRISPR and restriction modifications have evolved, phages have evolved several ways to overcome these. In terms of restriction endonucleases, there are several active and passive ways through which phage avoid cleavage. Passive mechanisms include abundance, spacing and orientation of restriction sites. Active mechanisms are more specific and in most cases include specific viral proteins have evolved to either inhibit restriction site recognition or proper REase activity 3.
To overcome the CRISPR-Cas bacterial defense, phages have evolved both simple and complex mechanisms. In certain cases, a simple point mutation in the PAM avoids acquisition of spacer sequence by Cas enzymes. Sometimes the whole prospacer and/or PAM site is deleted from the viral genome, as long as the deletion doesn’t significantly impair the phage replication cycle. Other phages harbour complex anti-CRIPSR proteins encoded in their genome. It seems like these proteins inhibit cleavage of Cas enzymes by preventing proper Cas-crRNA complex formation. Also, recent studies suggest phages have evolved a CRISPR-Cas system themselves. So far, these phage CRISPR-Cas system seem to form a Cas-crRNA in a similar fashion as the bacterial one, which can then deactivate the bacterial CRISPR defense system3.
The outer-membrane of Ignicoccus species was found to be composed of various derivatives of the typical lipid archaeol, including the derivative known as caldarchaeol [5] . The outer-membrane is dominated by a pore composed of the Imp1227 protein (Ignicoccus outer membrane protein 1227). The Imp1227 protein forms a large nonamer ring with a predicted pore size of 2nm[7] .
Application of Defense Systems in Biotechnology
CRISPR and restriction modification are defense mechanisms to phage infection which have vast applications in molecular biology and biotechnology. Restriction endonucleases are powerful tools in molecular biology and several specific fields, such as metabolic engineering, could have not been imagined without restriction enzymes. Restriction enzymes of the type II mechanism are the most common in laboratory applications and they effectively enable manipulation of foreign DNA through site specific cleavage9
The function of the CRISPR-Cas system is a fairly new discovery, and there are already several different applications it is used for. Most notably, the CRISPR-Cas system is an advanced and novel approach in genome engineering. The specificity of the CRISPR system allows screening for a desired mutations within genomes and occurs through crRNA:Cas directed cleavage at targeted sites9. The CRISPR-Cas system could also be used to artificially immunize bacterial strains against specific phages. This has many potential applications in the food industry, as many processes are dependent on bacteria, such as the dairy industry. In such industries, engineered phage immunity could decrease large economic losses that are caused by phage mediated infections 10. The CRISPR-Cas has many applications, and the future of this site specific nuclease will undoubtedly provide much more biotechnological advancement in the future.
Ignicoccus species are chemolithoautotrophs that use molecular hydrogen as the inorganic electron donor and elemental sulphur as the inorganic terminal electron acceptor[1] . The reduction of the elemental sulphur results in the production of hydrogen sulphide gas.
Ignicoccus are autotrophs in that they fix their own carbon dioxide into organic molecules. The carbon dioxide fixation process they use is a novel process called a dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle that involves 14 different enzymes[8] .
Members of
the Ignicoccus genus are able to use ammonium as a nitrogen source.
Growth Conditions
Because members of the Ignicoccus genus are hyperthermophiles and obligate anaerobes, it is not surprising that their growth conditions are very complex. They are grown in a liquid medium known as ½ SME Ignicoccus which is a solution of synthetic sea water which is then made anaerobic.
Grown in this media at their optimal growth temperature of 90C, the members of the Ignicoccus genus typically reach a cell density of ~4x107cells/mL[1] .
The addition of yeast extract to the ½ SME media has been shown to stimulate the growth and increase maximum cell density achieved. The mechanism by which this is achieved is not known[1] .
Symbiosis
Ignicoccus hospitalis is the only member of the genus Ignicoccus that has been shown to have an extensive symbiotic relationship with another organism.
Ignicoccus hospitalis has been shown to engage in symbiosis with Nanoarchaeum equitans . Nanoarchaeum equitans is a very small coccoid species with a cell diameter of 0.4 µm[9] . Genome analysis has provided much of the known information about this species.
To further complicate the symbiotic relationship between both species, it’s been observed that the presence of Nanoarchaeum equitans on the surface of Ignicoccus hospitalis somehow inhibits the cell replication of Ignicoccus hospitalis . How or why this occurs has not yet been elucidated[3] .
Nanoarchaeum equitans
Nanoarchaeum equitans has the smallest non-viral genome ever sequenced at 491kb[9] . Analysis of the genome sequence indicates that 95% of the predicted proteins and stable RNA molecules are somehow involved in repair and replication of the cell and its genome[3] .
Analysis of the genome also showed that Nanoarchaeum equitans lacks nearly all genes known to be required in amino acid, nucleotide, cofactor and lipid metabolism. This is partially supported by the evidence that Nanoarchaeum equitans has been shown to derive its cell membrane from its host Ignicoccus hospitalis cell membrane. The direct contact observed between Nanoarchaeum equitans and Ignicoccus hospitalis is hypothesized to form a pore between the two organisms in order to exchange metabolites or substrates (likely from Ignicoccus hospitalis towards Nanoarchaeum equitans due to the parasitic relationship). The exchange of periplasmic vesicles is not thought to be involved in metabolite or substrate exchange despite the presence of vesicles in the periplasm of Ignicoccus hospitalis .
These analyses of the Nanoarchaeum equitans genome support the fact of the extensive symbiotic relationship between Nanoarchaeum equitans and Ignicoccus hospitalis. However, it has not yet been proven that it is a strictly parasitic relationship and further research may prove that there is a commensal relationship between the two species.
References
(1) Westra, E. R. et al. The CRISPRs, they are a-changin’: how prokaryotes generate adaptive immunity. Annu. Rev. Genet. 46, 311–39 (2012). (2) Marcó, M. B., Moineau, S. & Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2, 149–158 (2012). (3) Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–87 (2013). (4) Szalay, A. A., Mackey, C. J. & Langridge, W. H. R. Restriction endonucleases and their applications JlJIIEIE. 1, 154–164 (1979). (5) Types of Restriction Endonucleases. (6) Williams, R. J. Restriction Endonucleases. 23, (2003). (7) Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–12 (2007). (8) Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–21 (2012). (9) Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. a. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–9 (2013). (10) Brüssow, H. Phages of Dairy bacteria. 283–303 (2001).