Batrachochytrium dendrobatidis
Introduction
Fungal disease has emerged as an increasing threat to a number of different organisms, especially amphibian species. Batrachochytrium dendrobatidis, a chytrid fungus that causes the infectious disease chytridiomycosis has been found to be the major cause of amphibian death caused by fungal infection. B. dendrobatidis infects the superficial, keratin-containing layers of amphibian skin. The infection then spreads across the skin, causing it to thicken and slough off. This thickening interferes with osmoregulation and eventually leads to death1. It has been found to affect at least 93 amphibian species in frogs, toads (Anura) and salamanders (Caudata)2. Evidence of B. dendrobatidis has been found worldwide and is believed to have originated in Africa and spread through the trade of Xenopus laevis3. B. dendrobatidis has been responsible for the epidemic illness and death of amphibian populations in Australia, New Zealand, Europe, Central America, and the United States4.
Batrachochytrium dendrobatidis
Physiology
Temperature has an effect on mortality rates associated with B. dendrobatidis. B. dendrobatidis can grows in 4-25oC, but grows optimally in 17-25oC. Nitrogen has a strong effect on the growth of B. dendrobatidis. It is possible that B. dendrobatidis grow in keratinized epidermal cells because they are dead and easier to invade 5.
Morphology
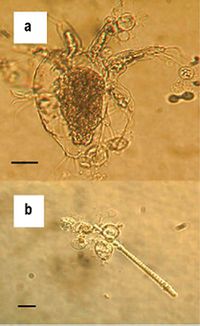
B. dendrobatidis is identified by its intracellular flask-shaped sporangia (spore containing bodies) and septate thalli. The fungus produces smooth-walled zoosporangia (zoospore containing bodies), which are generally spherical in shape. Each zoosporangium (5.2 ± 0.72 μm6) produces a single discharge tube, which penetrates the skin and releases mature zoospores. The zoospores (3-5 μm in diameter) are oval shaped and have a single posterior flagellum (19–20 μm in length), which allows it to move in water. There is little morphological difference in B. dendrobatidis strains4,7
Life Cycle
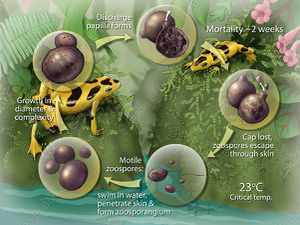
B. dendrobatidis progresses from a zoospore to a thallus (growing organism), which then produces a single zoosporangium (container for zoospores). The sporangium (contents of the zoosporangium) divides into new zoospores that exit the sporangium through one or more papillae. B. dendrobatidis has not been shown to reproduce sexually. The duration of the life cycle in vitro is 4 to 5 d at 22°C and due to the similarity in life cycles in vivo and in vitro, duration is assumed to be the same in both. B. dendrobatidis infects the superficial epidermis (stratum granulosum and stratum corneum) in amphibians. Immature sporangia are found in deeper cells, while mature zoosporangia and empty sporangia are found in the outer keratinized layers of the skin. Zoospores are released into the environment by discharge tubes that project out of the skin surface8.
Pathogen
Chytridiomycosis
B. dendrobatidis infects superficial layers of the skin in amphibians which contain keratin. During metamorphosis skin becomes increasingly keratinized, which allows the infection to spread over the organism. In juvaniles and adults the digits and pelvic patch of skin involved in osmoregulation are the most effected areas. As the infection continues the skin thickens and sloughs off. The skin in amphibians is intimately tied to regulation of hydration state, which can have compounding negative effects on the organism. The cause of death due to B. dendrobatidis, is cardiac arrest due to improper electrolyte blood levels9. Mortality rate and time till death by B. dendrobatidis varies with fungal dose, temperature, age of amphibian, and host species1,10
Spread
B. dendrobatidis originated in Africa and was transported to other countries through global trade of Xenopus laevis3. Infection has been documented in amphibians from exposure to infected soil or water. B. dendrobatidis can survive in tap water for 3 weeks and deionized water for 4 weeks. In lake water infectivity was observed for 7 weeks after introduction4. The bullfrog is the most commonly farmed amphibian and accidental introduction of captive bullfrogs into the wild populations is very common. B. dendrobatidis is asymptomatic in bullfrogs, making it hard to detect without performing tests. B. dendrobatidis has been detected in all introduced species of bullfrogs excluding Japan, and native bullfrogs from Eastern Canada11.
Methods of Control
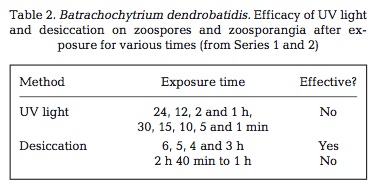
A number of physical and chemical disinfectants can cause 100% mortality in B. dendrobatidis. The fungus is highly sensitive to heat and died within 4 hours at 37oC. Desiccation also proved successful. UV light exposure did not kill 100% of the B. dendrobatidis for any of the tested exposure times. Many chemical methods proved useful. Ethanol and Virkon both killed 100% of B. dendrobatidis within 30 seconds. Sodium hyperchlorite was useful, as was Didecyldimethylammonium Chloride (DDAC). DDAC is promoted as being environmentally safe and is used in agriculture and in forestry. Dithane and Formaldehyde were not as effective. Sodium chloride and potassium permanganate were also not as effective12.
Conclusion
The IUCN has called amphibian chytridiomycosis “the worst infectious disease ever recorded among vertebrates in terms of the number of species impacted, and its propensity to drive them to extinction.” Although there are antifungal treatments available for organisms infected with B. dendrobatidis in captivity, there remains no solution to the problem presented by the spread of B. dendrobatidis in the wild. The incredible speed with which chytridiomycosis caused by B. dendrobatidis can damage a population and the widespread prevalence of this fungus adds to the difficulty in attempts to control further infection and aid already infected organisms. Many unique and invaluable amphibians species are at risk from not only this fungus, but from habitat loss, and urgent conservation efforts would benefit worldwide amphibian populations.
References
Edited by Claire Forster