Beggiatoa: Focus on Current Research
1. Classification
a. Higher order taxa
Domain: Bacteria
Phylum: Proteobacteria
Class: Gammaproteobacteria
Order: Thiotrichales
Family: Thiotrichaceae
Genus: Beggiatoa
2. Description and significance
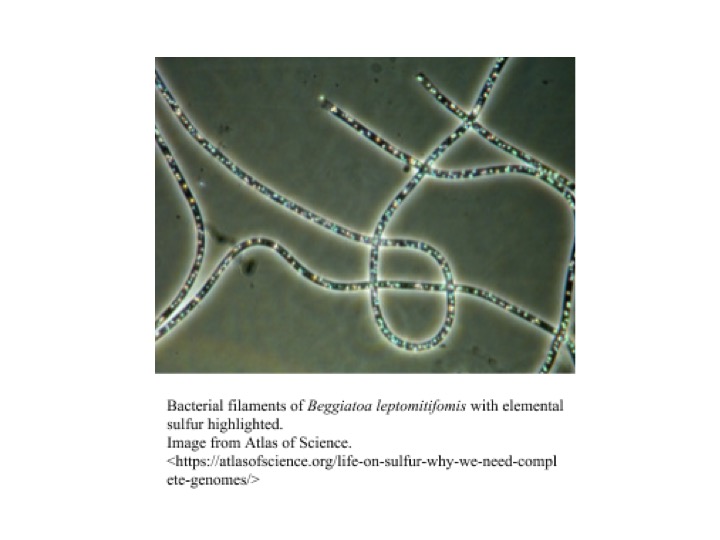
Beggiatoa is a genus of colorless, filament-forming bacteria with great morphological and metabolic diversity, that occupies a wide range of habitats. They can be cylindrical or disk-shaped, ranging 1-200 µm in width and 2-10 µm in length, and can form filaments up to hundreds of cells long [[[1]]]. Beggiatoa can move relatively rapidly by gliding motility and can use this motility to position themselves at optimal nutrient concentrations and into and out of sediments in response to light, as they exhibit photophobic behavior [[[1]]][[[2]]]. Beggiatoa are classified into three subgroups. These subgroups include (1) thin, heterotrophic, freshwater strains; (2) thin, obligately or facultatively chemolithotrophic, marine strains; and (3) wide, autotrophic, vacuolated, marine strains [[[1]]]. However, there are only two classified species of Beggiatoa: Beggiatoa alba and Beggiatoa leptomitoformis [[[3]]].
Beggiatoa is most noted for its ability to oxidize sulfur, being the first organism discovered to perform chemolithotrophy, the use of inorganic compounds as an energy source [[[4]]]. Because of this ability, Beggiatoa ssp. tend to form mats with layers of bacteria adapted to the specific sulfide and oxygen concentration gradient [[[2]]].
Beggiatoa is currently of increasing interest to researchers due to its applications in medicine. However, while interest continues to grow in the genus, its genome remains not well-characterized. Additionally, there is a lack of information for classification below the genus level.
a. Discovery
The initial discovery of Beggiatoa dates back to 1842, where it was named after Italian medic and botanist F.S. Beggiato [[[5]]]. The organism lives in areas rich in sulfur, like deep sea basins of the Baltic Sea, but have more recently been isolated in freshwater sediments of Baton Rouge, Louisiana as well. [[[6]]][[[7]]] In 1887, Sergei Winogradsky--a Russian botanist-- found that Beggiatoa, specifically Beggiatoa alba, used hydrogen sulfide (H2S) as an energy source through oxidation. While not credited for the discovery of Beggiatoa, Winogradsky’s finding is referred to as the first discovery of lithotrophy [[[4]]]. The second known Beggiatoa species, Beggiatoa leptomitiformis, has been studied since 1978 and is also found in similar environments as Beggiatoa alba. Most recently in 2017, Beggiatoa leptomitiformis has been isolated from a freshwater spring contaminated with wastewater in Moscow, Russia [[[3]]].
b. Genome Structure
The genome sequence of Beggiatoa is not yet fully mapped, but research has been done to observe its basic structure within the organism. Like many prokaryotes, Beggiatoa has a singular circular DNA chromosome, but it is not known how this DNA structure is organized. It is estimated, from a consensus optical map of a single circular chromosome, that the size of the genome is 7.4Mb. The GC content percentage (averaged from three strains) ranges from 40.0 to 42.7 [8]. Through a 16S rRNA sequence similarity analysis, the closest relatives to Beggiatoa are Nitrosococcus oceani and Methylococcus capsulatus, both of which have fully sequenced genomes [[[9]]]. Some attempts to sequence the genome have been made, but were not successful in fully mapping the genome. This is due to the difficulty of isolating, purifying, and growing the bacterium in vivo. The two main techniques used to partially map the genome are pyrosequencing and whole genome amplification (WGA). Reads from repeat regions were unusually high, but it is unclear if that is due to repetitive DNA content or an artifact of WGA [[[9]]].
c. Metabolism
Beggiatoa are most abundant as sulfur and nitrogen cyclers and have been historically categorized as chemolithotrophs [[[10]]]. However, the metabolism of the genus ranges across various combinations of chemo-, auto- and heterotrophy, litho- and organotrophy depending on sulfur and oxygen concentrations in its surroundings. The bacteria typically resides in aquatic environments at the transition between oxic and sulfonic zones in porous sediments with sufficient space for motility [[[10]]].
Most strains participate in the oxidation of inorganic sulfur, coupling it with oxygen respiration. In the presence of oxygen, the bacteria oxidizes hydrogen sulfide to elemental sulfur with the help of Fe(III). Sulfur products are incorporated into vacuoles in their cell membrane, giving Beggiatoa mats their white appearance [[[2]]]. Some Beggiatoa have the ability to anaerobically oxidize sulfur with internally stored nitrate if oxygen presence is low. The bacteria’s capacity to store sulfur and nitrogen allow it to survive in suboxic areas, between the O2 and H2S interface. This way, Beggiatoa can grow where neither sulfide nor oxygen is found, giving them an advantage over other sulfide oxidizing bacteria [[[9]]]. Through gliding motility the bacteria is able to relocate in unstable chemical surroundings. The flexibility in their metabolism suggests that Beggiatoa has developed a very capable chemotaxis to adjust to a variable microenvironment. In addition to chemolithotrophy, a marine strain of Beggiatoa alba has been observed to be chemoautotrophic, growing on acetate in absence of reduced sulfur [[[11]]]. In a study conducted by Nelson et al. in 2008, two strains of freshwater Beggiatoa were observed to be methylotrophs, being able to use methanol as a sole carbon source under microoxic conditions [[[8]]].
d. Cell Structure
Beggiatoa can range in size and shape, from thin, cylindrical-shaped to wide disk-shaped [[[1]]]. Cells occur in filaments up to 100 or more cells long, and these filaments can occur singly or within mats [[[1]]]. These filaments also secrete a polysaccharide slime matrix on which they can perform gliding motility [[[12]]]. Beggiatoa stain Gram-negative, but have a more complex cell envelope than typical Gram-negative bacteria, with up to five additional cell walls outside of the cytoplasmic membrane and peptidoglycan layer [[[12]]]. In the presence of sulfide, Beggiatoa form sulfur granules within the cell envelope, a process associated with its sulfur-oxidizing capabilities [[[1]]][[[12]]].
e. Ecology
Beggiatoa survive in a wide variety of marine and freshwater aquatic habitats, from lakes and streams, to estuaries and coastal zones, as well as in polluted waters [[[12]]]. Beggiatoa are generally mesophilic (prefer moderate temperatures), but some strains have been found to grow at temperatures as low as 0°C and as high as 72°C [[[12]]]. Beggiatoa are concentrated in sulfur-rich sediment zones and can grow in both oxic and anoxic conditions [[[13]]]. Because of this they tend to aggregate as white, filamentous mats at the sulfur-oxygen interface [[[13]]].
3. Modern Research and Medical Applications
a. Insulin Regulation
One recent study explored the potential association between Beggiatoa’s Photoactivatable Adenylyl Cyclase (PAC) and human insulin production. In humans, pancreatic Beta cells are responsible for producing insulin, a hormone that enables the usage of sugar from food, which is synthesized and secreted by a complex intracellular pathway that includes the molecule cyclic AMP (cAMP). cAMP plays an important role in metabolic regulation of cells. The role of cAMP is to promote the release of insulin from pancreatic beta cells so that sugar may be broken down in metabolism. In Type I Diabetes, insulin-producing pancreatic Beta cells are destroyed by Beta-cell specific autoimmune processes [[[9]]], which corresponds to decreased expression of cAMP. Decreased cAMP expression causes a decreased production of insulin, resulting in high blood sugar content seen in diabetics. Using human Beta-cells in culture, it was found that employment of Beggiatoa’s PAC with light mitigated cAMP levels and therefore increased insulin production and secretion levels. Photostimulation of PAC yielded rises in cAMP levels that were noted after only 5 minutes of exposure, and which dropped significantly 12 minutes post-illumination. Greater insulin release was observed over repeated cycles of photostimulation without adverse effects on growth of Beta-cells. These findings are significant to both the understanding of Type I Diabetic mechanisms and the progress of therapy development for Type I Diabetes in humans[[[14]]].
b. Motility Analysis for Pathogenesis
In a pair of recent studies, researches took interest in the motility mechanism of Beggiatoa. Changes in motility patterns were observed under low oxygen conditions [[[15]]] and various temperature and pressure ranges [[[16]]]. Beggiatoa is capable of what’s known as gliding motility, which is most commonly seen among cells of bacterial mats or biofilms, where bacteria do not need to use external flagella for movement. The mechanisms of gliding motility are still fairly unknown, where some bacteria “glide” using pili motions similar to twitching motility, while others don’t use pili or flagella at all. Although the exact mechanism of gliding motility is a subject of speculation, it is understood that this type of motility actually allows for cell-to-cell binding and communication for the formation of biofilms, mats, and other similar microbial communities. Gliding motility is frequently observed in infectious bacteria and human pathogens, where biofilm formation allows for protected growth of the infection, while also permitting bacterium to infect individual cells. These two studies found that Beggiatoa enzymatically control their own motility in response to decreased oxygen levels, and altering pressures and temperatures. [[[15]]][[[16]]]. While Beggiatoa may not be directly pathogenic to humans, the findings of these studies could give insight into the mechanisms gliding motility used by infectious bacteria, as a basis for future therapeutic development.
3. References
[1] Garrity G.M., Bell J.A., Lilburn T. Thiotrichalesord. nov. . In: Brenner D.J. et al. (eds) Bergey’s Manual of Systematic Bacteriology 2005. Springer, Boston, MA.
[2] Preisler, A., D. de Beer, A. Lichtschlag, G. Lavik, A. Boetius, and B. B. Jørgensen. 2007. Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. ISMEJ.1: 341–53. doi: 10.1038/ismej.2007.50
[3] Dubinina G, Savvichev A, Orlova M, Gavrish E, Verbarg S, Grabovich M. Beggiatoa leptomitoformis sp. nov., the first freshwater member of the genus capable of chemolithoautotrophic growth. Int J Syst Evol Microbiol 67(2):197-204 doi:10.1099/ijsem.0.001584
[4] Dworkin. Sergei Winogradsky: a founder of modern microbiology and the first microbial ecologist. FEMS. 2011; 36(1) 364-379.
[5] Trevisan, V. Prospetto della Flora Euganea. Coi Tipi Del Seminario, 1842. Padova, pp. 1-68
[6] Mezzino M, Strohl, W, Larkin, J. Characterization of Beggiatoa alba. Archives of Microbiology. 1984;137(2) 139-144.
[7] Yücel M, Sommer S, Dale AW, Pfannkuche O. Microbial Sulfide Filter along a Benthic Redox Gradient in the Eastern Gotland Basin, Baltic Sea. Frontiers in Microbiology. 2017;8:169. doi:10.3389/fmicb.2017.00169.
[8] Mezzino, M.J., Strohl, W.R. & Larkin, J.M. Methylotrophy in Freshwater Beggiatoa alba Strains. Arch Microbiol 1984. 137: 139. https://doi.org/10.1007/BF00414455
[9] Mußmann M, Hu FZ, Richter M, et al. Insights into the Genome of Large Sulfur Bacteria Revealed by Analysis of Single Filaments. Moran NA, ed. PLoS Biology. 2007;5(9):e230. doi:10.1371/journal.pbio.0050230
[10] Jørgensen, B. B., & Revsbech, N. P. Colorless Sulfur Bacteria, Beggiatoa spp. and Thiovulum spp., in O2 and H2S Microgradients. Applied and Environmental Microbiology, 45(4), 1261–1270.
[11] Nelson, D. C., and H. W. Jannasch. 1983. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient cultures. Arch. Microbiol. 136:262-269.
[12] Larkin, J.M. and Strohl, W.R. 1983. Beggiatoa, Thiothrix, and Thioploca. Ann. Rev. Microbiol. 37:341-367.
[13] Jørgensen, B. B. Ecology of the bacteria of the sulfur cycle with special reference to anoxic-oxic interface environments. Phil. Trans. R. Soc. Lond. B 298:543-561.
[14] Ji-Won Yoon, Hee-Sook Jun. Autoimmune Destruction of Pancreatic Beta Cells. Am J Ther. 2005 Nov-Dec; 12(6): 580–591. Accessed: https://www.ncbi.nlm.nih.gov/pubmed/16280652
[15] Zhang F, Tzanakakis ES. Optogenetic regulation of insulin secretion in pancreatic β-cells. Scientific Reports. 2017;7:9357. doi:10.1038/s41598-017-09937-0.
[16] Dunker, R., Røy, H., Kamp, A., & Jørgensen, B. B. Motility patterns of filamentous sulfur bacteria, Beggiatoa spp. Retrieved October 05, 2017, from http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6941.2011.01099.x/full