Borrelia burgdorferi: Difference between revisions
| Line 85: | Line 85: | ||
Current research is also investigating other mechanisms in which ''Borrelia burgdorferi'' is able to evade the host organisms immune response. One theory is that the bacterial outer surface proteins are able to bind to host factor H, a regulator in the complement activation pathway. By binding to this regulator, the bacteria may be able to protect themselves from complement killing. Researchers tested this theory by developing strains of mice that were deficient in host factor H in order to see whether ''B. burgdorferi'' binding was necessary for infection. Their results showed that the deficient mice were infected at similar levels to wild-type mice. In addition, control experiments showed that many of the bacteria did not bind to host factor H even when they had outer surface proteins present capable of doing so. They also showed that some mice deficient in complement activating pathways were able to contain the ''Borrelia'' infection. These results indicate that ''B. burgdorferi'' must have other mechanisms to prevent complement activation in the host, and that complement mediated killing may not be the only way for the host to combat the bacteria. The results of these experiments help us better understand the mechanisms involved in ''B. burgdorferi'' infections (Woodman). | Current research is also investigating other mechanisms in which ''Borrelia burgdorferi'' is able to evade the host organisms immune response. One theory is that the bacterial outer surface proteins are able to bind to host factor H, a regulator in the complement activation pathway. By binding to this regulator, the bacteria may be able to protect themselves from complement killing. Researchers tested this theory by developing strains of mice that were deficient in host factor H in order to see whether ''B. burgdorferi'' binding was necessary for infection. Their results showed that the deficient mice were infected at similar levels to wild-type mice. In addition, control experiments showed that many of the bacteria did not bind to host factor H even when they had outer surface proteins present capable of doing so. They also showed that some mice deficient in complement activating pathways were able to contain the ''Borrelia'' infection. These results indicate that ''B. burgdorferi'' must have other mechanisms to prevent complement activation in the host, and that complement mediated killing may not be the only way for the host to combat the bacteria. The results of these experiments help us better understand the mechanisms involved in ''B. burgdorferi'' infections (Woodman). | ||
Interesting research was also performed concerning the sensitivity of certain individuals to tick bites and whether this sensitivity could limit the ability of ''B. burgdorferi'' to infect that person. The researchers sampled a population in Rhode Island and determined that people who had more exposure to tick bites had stronger and faster reactions to the bites and also had less risk of developing Lyme disease. Their results showed that people who were frequently bitten by ticks developed an immunity against them, possibly against tick salivary antigens. Therefore, the new hypersensitivity may be able to protect this individuals from ''Borrelia'' infection and help them recognize tick bites and remove them before the bacteria can be transmitted. These results lend support to the idea that immunity against tick salivary proteins may help prevent infection by ''B. burgdorferi'' and therefore the symptoms of Lyme disease (Burke). | |||
==References== | ==References== | ||
Revision as of 03:58, 5 June 2007
A Microbial Biorealm page on the genus Borrelia burgdorferi
Classification
Higher order taxa
Bacteria; Spirochaetes (Phylum); Spirochaetes (Class); Spirochaetales; Spirochaetaceae
Species
Borrelia burgdorferi
|
NCBI: Taxonomy |
Description and significance
Borrelia burgdorferi is a Gram-negative spirochete bacteria that is well known as the causative agent of Lyme disease. In 1982, a few years after Lyme disease was first diagnosed, it was determined that B. burgdorferi was being transmitted to humans by ticks. Since then, it's genome has been fully sequenced and researchers have been able to study the interesting mechanism of host and vector transmission that the bacteria has adopted. However, the exact mechanisms for it's pathology are still trying to be understood. B. burgdorferi is currently being used to answer interesting questions surrounding vector transmission, host adaptation and plasmid maintenance (Rosa). According to the Center for Disease Control, Lyme disease caused by B. burgdorferi has become the most common vector-borne bacterial disease in the world. B. burgdorferi's unique structure and adaptations allow it to be an effective invasive pathogen and an important organism to research.
Describe the appearance, habitat, etc. of the organism, and why it is important enough to have its genome sequenced. Describe how and where it was isolated. Include a picture or two (with sources) if you can find them.
Genome structure
B. burgdorferi has a very unique genome. It consists of one large linear chromosome that is 910,725 base pairs long. This chromosome contains approximately 853 genes coding for basic functions such as DNA replication, transcription, translation as well as transport and metabolic systems. However, in addition to this chromosome, the bacterium also contains 21 other linear and circular plasmids that add an additional 533,000 base pairs of DNA. The genomic organization of B. burgdorferi is unique due to the high number of plasmids. Research has shown that some bacteria lacking a complete set of plasmids are unable to successfully infect their host, leading researchers to believe that the plasmids may encode virulent DNA (Fraser). One plasmid named lp25 has been found to be necessary for Borrelia infection. An lp25 gene named BBE22 has been discovered to encode for a nicotinamidase which by itself can maintain the ability for the bacteria to infect the host even when the plasmid is removed (Purser). However, in general, the sequenced genome does not contain any obvious genes coding for pathogenesis, and therefore, the mechanisms of B. burgdorferi infections are still a hot topic of research.
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Does it have any plasmids? Are they important to the organism's lifestyle?
Cell structure and metabolism
B. burgdorferi is a helical shaped spirochete bacterium. It has an inner and outer membrane as well as a flexible cell wall. Inside the bacteria's cell membranes is the protoplasm, which, due to the spiral shape of the bacteria, is long and cylindrical. The cell is normally only 1 μm wide but can be 10-25 μm long. Spirochete bacteria such as B. burgdorferi have a unique structural characteristic. The cell's flagella are located inside the periplasm between the inner and outer cell membranes. The interactions between the flagella and cell cylinder allows the cell to travel through highly viscous fluids and materials (Charon). This adaptation is especially critical for the ability of B. burgdorferi to travel through the tissue of it's infected host or vector, causing it to be highly invasive.
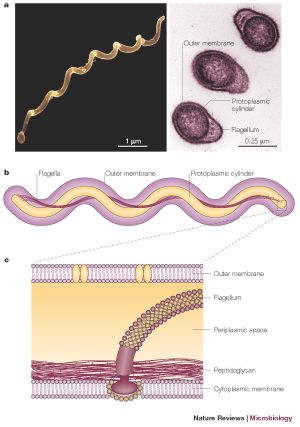
Another important cellular component of B. burgdorferi is it's outer surface proteins. Researchers have investigated a variety of outer membrane surface proteins (Osp) and have found them to play important roles in transmission of the bacteria. The cell expresses different surface proteins, most notably OspA and OspC. However, they are not seen on all cells. When the bacteria is inside of an unfed tick, it produces mostly OspA. However, the bacteria that are inside of infected mammals express mostly OspC (Schwan). This has caused speculation that the bacteria produces specific outer surface proteins in order to successfully invade and inhabit certain organisms. For example, OspA may function to retain the bacteria in the tick stomach, while the presence of OspC may encourage the bacteria to enter the salivary glands and enter the host (Ohnishi). OspA, OspC, and other outer surface proteins may play important roles in the ability of the bacteria to infect and survive inside their hosts.
The genome sequencing of B. burgdorferi showed that it has genes for metabolism similar to other parasites. These bacteria have limited metabolic capabilities and rely on their host for energy precursors. The genome of B. burgdorferi encodes many transport proteins such as ABC transporters and enyzymes and proteins used in the phosphotransferase system. These transport systems are likely used to obtain compounds from the host serum or environment. B. burgdorferi uses glucose and other similar carbohydrates as it's main energy source in mammals. In ticks, it is believed that N-acetylglucosamine (NAG) is a major energy source as it is a main component of tick cuticle and is essential for in vitro growth of the bacteria. B. burgdorferi does not contain genes or enzymes for the TCA cycle, oxidative phosphorylation or an electron transport chain. Instead, it produces ATP solely by using substrate-level phosphorylation and achieves reducing power using the pentose phosphate pathway (Fraser).
Describe any interesting features and/or cell structures; how it gains energy; what important molecules it produces.
Ecology
Borrelia burgdorferi is maintained in a natural cycle of infection by ticks. The ticks acquire and transmit the bacteria by feeding on a variety of small mammals and birds that act as a reservoir host for the Borrelia. B. burgdorferi is transmitted specifically by ticks of the genus Ixodes, which include a variety of different species found in different geographical locations. Ixodes scapularis is the most common vector and is found in the Northeastern US. Ixodes pacificus is found on the west coast, while species Ixodes ricinus and persulcatus are found in Europe and Asia. These ticks acquire and transmit the bacteria during three distinct feeding phases of their life cycle; larval, nymphal and adult stages.
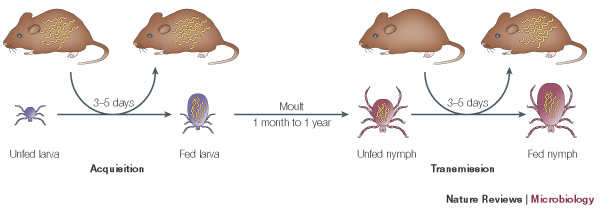
Describe any interactions with other organisms (included eukaryotes), contributions to the environment, effect on environment, etc.
Pathology
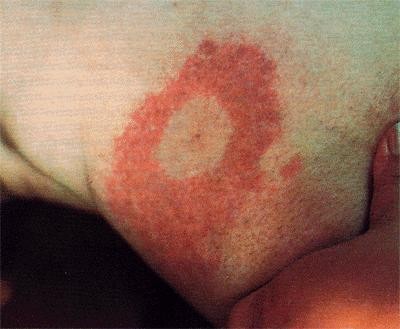
Lyme disease was first identified due to an outbreak of juvenile rheumatoid arthritis. This is one of the later stage symptoms of the B. burgdorferi infection. Ticks use many small mammals to harbor the bacteria, and humans only become inadvertently infected when bitten. The first sign of infection is the Erythema migrans, a circular rash at the site of the tick bite that appears after a few days delay. From there, infected humans may develop similar rashes on other sites of the body as well as symptoms such as fatigue, fever, headache, and muscle and joint aches. If the infection spreads throughout the body it has reached Stage II of the infection, which is marked by symptoms such as facial palsy, meningitis, extreme joint pain and heart palpitations. If it is left untreated for a few months the infection will reach Stage III and patients may develop arthritis as well as severe joint pain and swelling ("Lyme Disease," Wormser). For more on diagnosis and treatment of Lyme disease, refer to the CDC website on Lyme disease.
How does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
Application to Biotechnology
Does this organism produce any useful compounds or enzymes? What are they and how are they used?
Current Research
Much of the current research surrounding B. burgdorferi concentrates on discovering the mechanisms through which the bacteria invades the human body in order to produce more effective treatments against it. As a member of the Spirocheate phylum, B. burgdorferi is quite distinct from many other bacterial groups and therefore many of the common genetic tools do not work on the bacteria. It is also very difficult to transform in the laboratory.
The most current research of B. burgdorferi surrounds the discovery of the immunosuppressive tick salivary protein, Salp15. This protein is believed to be involved in the transmission of the bacteria from the vector to the host. It is believed that the tick produces Salp15, which binds to B. burgdorferi and protects it from the host antibody response (thus earning it's immunosuppressive title). This makes Salp15 an attractive target for research and possible use in vaccines ("Transmission").
In April 2007, Juncadella and colleagues determined that Salp15 inhibited T-cell activation by interfering with the binding with the CD4 receptors of T-cells and disrupting their signalling cascade. The researchers were able to identify the C-terminal peptide of Salp15 that is responsible for it's immunosuppressive properties. They confirmed that the Salp15-CD4 interactions caused premature activation and a defective immune response. These results can help future researchers uncover methods to block the activity of Salp15 and possibly develop an effective vaccine.
Current research is also investigating other mechanisms in which Borrelia burgdorferi is able to evade the host organisms immune response. One theory is that the bacterial outer surface proteins are able to bind to host factor H, a regulator in the complement activation pathway. By binding to this regulator, the bacteria may be able to protect themselves from complement killing. Researchers tested this theory by developing strains of mice that were deficient in host factor H in order to see whether B. burgdorferi binding was necessary for infection. Their results showed that the deficient mice were infected at similar levels to wild-type mice. In addition, control experiments showed that many of the bacteria did not bind to host factor H even when they had outer surface proteins present capable of doing so. They also showed that some mice deficient in complement activating pathways were able to contain the Borrelia infection. These results indicate that B. burgdorferi must have other mechanisms to prevent complement activation in the host, and that complement mediated killing may not be the only way for the host to combat the bacteria. The results of these experiments help us better understand the mechanisms involved in B. burgdorferi infections (Woodman).
Interesting research was also performed concerning the sensitivity of certain individuals to tick bites and whether this sensitivity could limit the ability of B. burgdorferi to infect that person. The researchers sampled a population in Rhode Island and determined that people who had more exposure to tick bites had stronger and faster reactions to the bites and also had less risk of developing Lyme disease. Their results showed that people who were frequently bitten by ticks developed an immunity against them, possibly against tick salivary antigens. Therefore, the new hypersensitivity may be able to protect this individuals from Borrelia infection and help them recognize tick bites and remove them before the bacteria can be transmitted. These results lend support to the idea that immunity against tick salivary proteins may help prevent infection by B. burgdorferi and therefore the symptoms of Lyme disease (Burke).
References
"Lyme Disease." Center For Disease Control: Division of Vector-Borne Infectious Diseases.
Edited by Dan Holligan, student of Rachel Larsen and Kit Pogliano at UCSD.