Borrelia burgdorferi and Lyme Disease Detection: Difference between revisions
| Line 32: | Line 32: | ||
==Life Cycle== | ==Life Cycle== | ||
[[File:rhg. | [[File:rhg.webp|thumb|300px|right|Figure #- ADD HERE CAPTION https://www.nature.com/articles/s41579-020-0400-5]] | ||
Tick life cycle- | Tick life cycle- | ||
Revision as of 20:37, 18 April 2022
Overview
By Roya Best
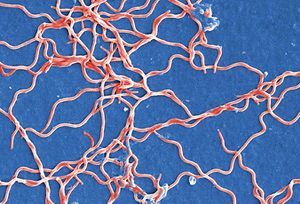
Borrelia burgdorferi is a bacterial eubacterial phylum spirochaete and a tick borne parasite.[1] It is one of the known causative agents for Lyme Disease.[1] Borrelia burgdorferi was named after Willy Burgdorfer who first isolated the bacteria in 1982.[2] The spirochete is a flat wave shape that is commonly 0.3 micrometers in width and ranges from 5 to 20 micrometers in length.[3] It has both an outer and inner membrane with a thin layer of peptidoglycan separating the membranes[3] Seven to eleven bundled periplasmic flagella reside within the membranes and allow the bacterium to move through a highly viscosity medium, which increases its virulence factor.[4] The flagellar filaments wrap around the cell and rotate in order to help the flagellar motor propel the bacteria in a signature corkscrew motion.[3] The doubling time of the bacteria ranges from 24 to 48 hours.[5] Borrelia burgdorferi is different from common pathogenic bacteria because it lacks the common virulence factors like toxins, a specialized secretion system, and lipopolysaccharides.[1] The bacteria lacks common biosynthetic abilities and heavily relies on its host for nutrients and other factors for its survival.[1]
Genetics
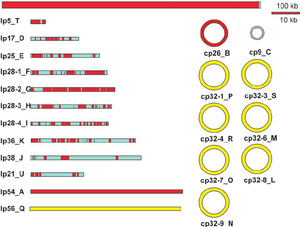
The genome of Borrelia burgdorferi is a linear chromosome with many smaller plasmids that are both linear and circular.[1] The linear chromosome is about 950 kb and the linear and circular plasmids range from 9 to 62 kb, which all encode 853 genes.[1][6] The genome of Borrelia burgdorferi was the third ever genome sequenced.[6] Linear chromosomes are not common in bacteria, however, in Borrelia burgdorferi it seems to provide an advantage that has continued to allow the linear chromosome to persist.[1] The Borrelia burgdorferi genetic sequence shows that the flagella are hidden between the two membranes compared to other organisms that have it externally radiating outward.[1] The flagella are strategically positioned in order to provide Borrelia burgdorferi an advantage in hiding from the host immune system, as this is critical in its survival.[1]
Borrelia burgdorferi lacks the classic genes to synthesize amino acids, fatty acids, enzyme cofactors, and nucleotides.[1] It takes advantage of its host and obtains these important materials.[1] The inability to synthesize these things is likely lost in coevolution with ticks and other hosts.[7] Additionally, Borrelia burgdorferi lacks the genes to use the citric acid cycle, oxidative phosphorylation, and cellular biosynthesis.[7][8] Borrelia burgdorferi, however, has the genes for and derives its energy from glycolysis and fermentation of sugars.[7]
Borrelia burgdorferi interacts with platelets, endothelial cells, chondrocytes, and extracellular matrix when infecting a host.[6] These interactions inhibit the proper function of various mechanisms in these infected areas. This leads to the symptoms of Lyme Disease.[6]
Lyme Disease History and Overview
Lyme Disease is an enzootic vector-borne disease that is transmitted by Ixodes tick.[8] Lyme Disease was first discovered by Doctor Alan Steere in 1977 when he noticed that people were being infected with an unknown pathogen in the same geographic location during the same time of the year presenting with the same symptoms.[1] The first confirmed case was in Lyme, Connecticut, thus providing the name of the Disease.[6] In 1981 it was finally discovered that Borrelia genus bacteria were the causative agents of Lyme Disease.[6]
There are a few Borrelia species that can cause Lyme Disease, however, Borrelia burgdorferi is the most common in the US.[1] Lyme Disease is the leading vector-borne disease in the US.[1] There are an estimated 476,000 cases each year of Lyme Disease in the US according to numbers from 2010 to 2018.[9] However, this is most likely an underestimate because Lyme Disease is difficult to diagnose.[9] Lyme Disease is endemic to the US, Europe, and Asia.[8] Most cases are reported during the Spring and Summer months due to the life cycle of the reservoir, the Ixodes ticks.[1][8] Common animals that can become infected with Borrelia burgdorferi are white-footed mice, chipmunks, dogs, white-tailed deer, squirrels, horses, opossums, and raccoons.[8]
Research surrounding Lyme Disease is sparse due to the lack of federal funding. The annual NIH investment in research is much lower than in other infectious diseases.[9] Thirty million dollars a year is allocated to Lyme Disease research which affects over 470,000 individuals annually.[9] However, 36 million dollars is allocated to West Nile Virus which only affects about 3,000 individuals and 202 million dollars is allocated to Malaria which only affects under 2,000 individuals.[9] The lack of funding surrounding Lyme Disease is highly controversial and why individuals with the disease commonly suffer due to improper diagnosis and must tolerate its symptoms.[9]
Transmission
Ticks become infected with Borrelia burgdorferi when they have a blood meal from an infected reservoir, such as a white-footed mouse or chipmunk.[1] When an infected tick bites an animal or human, Borrelia burgdorferi will migrate from the tick’s midgut to the salivary glands and then into the bloodstream of the new host.[1] The spirochete then travels from the bloodstream to various tissues within the new host.[1] The location in which Borrelia burgdorferi lands is where the most symptoms will be experienced.[1] While no virulence factors are found within Borrelia burgdorferi, symptoms will occur as a result of the inflammatory response and damage the bacteria causes when it replicates.[1] Ticks can attach to any part of the animal or human in order to take a blood meal and infect the individual with Borrelia burgdorferi.[10] However, on humans, ticks particularly like to attach to hard-to-see places.[10] These areas include behind the near, on the scalp, armpits, and behind the knees.[10]
Acquiring the disease depends on many factors, including, density, distribution, and prevalence of infected ticks in the individual's area.[8] Cases of Lyme Disease are the most common in the Northeast and Northern Central states within the US.[8] The most reported cases occur May through August due to the life cycle of ticks, tick feeding time, and increase in human activity outdoors.[8] A tick must be attached to its new host for over 36 hours in order to transmit the disease.[8] Adult ticks and nymph ticks can transmit the disease.[8] However, it is much more likely to be infected by a nymph tick because of its small size and the likelihood of remaining attached for over 36 hours.[8]
IMAGE IN lifecycle of blacklegged ticks- CDC transmission
Life Cycle

Tick life cycle- Ticks are the only known natural agents that can infect animals and humans with Borrelia burgdorferi.[1] The tick genus Ixodes can become infected with and transmit Borrelia burgdorferi between hosts and reservoirs.[1]
The life cycle of the Ixodes ticks heavily influences the transmission of Borrelia burgdorferi between various animals and humans.[1] The tick has a life span of two years and undergoes different life stages.[1] The egg phase of ticks has no influence on Lyme Disease and Borrelia burgdorferi can not be contracted during this stage.[8] The larva stage is when ticks must take a blood meal to survive and grow, thus feeding on small animals, usually mice.[8] This stage is critical to Lyme Disease as ticks can become infected with Borrelia burgdorferi.[8] The combination of taking a blood meal and feeding upon white-footed mice is key because white-footed mice are known as one of the main reservoirs for which ticks become infected with Borrelia burgdorferi.[8] The next stage of a tick’s life cycle is the nymph stage.[8] At this stage, ticks can infect others with Borrelia burgdorferi.[8] At the nymph stage, ticks also can infect small animals and humans.[8] Nymphs are extremely small in size and can evade detection by humans, thus being attached long enough to infect humans with Borrelia burgdorferi.[8] Ticks during the nymph stage feed in the spring and summer, when Lyme Disease cases are reported the highest.[8] After the nymph stage, ticks molt into adults.[8] Adult ticks can infect other animals and humans with Borrelia burgdorferi.[8] However, it is not common for adult ticks to transmit Borrelia burgdorferi to humans because of their large size, which allows them to be easily seen and removed.[8]
Lyme Disease life cycle- Lyme disease itself goes through a life cycle during the transmission of Borrelia burgdorferi between hosts and reservoirs.[7] Larva or nymphs acquire Borrelia burgdorferi during their first blood meal from an infected host.[7] Once infected, Borrelia burgdorferi persists in the tick’s midgut.[7] When the infected nymph bites an uninfected animal the bite triggers the Borrelia burgdorferi to replicate, escape from the tick’s midgut, and exit through the salivary glands of the tick to another host.[7] This life cycle of Borrelia burgdorferi and Lyme Disease thus completes the enzootic cycle.[7]
RED HAS GREAT FIGURE3 ADD. https://pubmed.ncbi.nlm.nih.gov/22974303/#&gid=article-figures&pid=figure-3-uid-2
Stages and Symptoms
There are three stages to Lyme Disease: early localized stage, early disseminated stage, and late disseminated stage.[11] With each stage, new symptoms develop, and old ones may persist or disappear.[11]
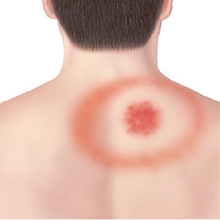
Stage 1- Early Localized Stage Early localized Lyme DIsease occurs three days to one month after the initial tick bite.[11] The local area of the bite will be the area that experiences the most symptoms.[11] About 70% of individuals will develop a “bull’s eye” rash.[11][8] This rash is characteristic of Lyme Disease and commonly leads to diagnosis.[11][8] It may get as large as 15 cm and last up to three weeks if untreated.[11][12] Symptoms at this stage include flu-like symptoms, such as fever, eye redness, neck stiffness, and headaches.[11][12] Antibiotics at this stage are the most effective and can prevent further growth of Borrelia burgdorferi and sometimes lead to being cured of the disease.[11]
Stage 2- Early Dissemination Stage The early dissemination stage of Lyme Disease occurs weeks to months after initial infection if left untreated.[11] At this point, Borrelia burgdorferi begins to spread throughout the bloodstream and begins to affect other parts of the body beyond the initial bite site.[11] General symptoms include fever, chills, fatigue, and lymphadenopathy.[11] The heart may begin to be affected and lead to arrhythmias or myocarditis.[11] About 4-8% of individuals experience cardiac symptoms.[8] The musculoskeletal system may also be affected and lead to arthritis development.[11] Arthritis is common with about 60% of those with Lyme Disease developing the symptom.[8] Additionally, the nervous system may begin to be affected and present with loss of memory or facial paralysis.[11] Neurological symptoms affect about 15-20% of individuals who are not treated.[8]
Stage 3- Late Dissemination Stage Late disseminated Lyme Disease occurs after one year of initial infection and is also considered chronic Lyme Disease.[11] Symptoms at this stage are long-term and may include encephalitis, meningitis, and severe arthritis.[11][12] Arthritis at this point in the disease is commonly harsh on individuals' knees.[12] Other symptoms from previous stages most likely would persist throughout this stage.[11]
Evasion and Detection
Treatment
When diagnosed early enough, Lyme Disease is treatable and curable.[8] Diagnoses that fall within the early stage of the disease is deemed most likely to be curable.[8] The antibiotics amoxicillin and doxycycline are commonly used to treat early detection and have not yet developed neurological symptoms.[13]A course of antibiotics is recommended to last 14 to 28 days depending on the medication, dosage, and individual.[8] For those with arthritis, which indicates late stages of Lyme Disease, a course of the aforementioned for 28 days is recommended.[9] Individuals with neurological symptoms are recommended to have treatment via intravenous ceftriaxone.[9]
If an individual has been previously infected with Borrelia burgdorferi and developed Lyme Disease it is possible to become infected again.[14] Those who were infected with late Lyme Disease are very unlikely to become reinfected with Lyme Disease again.[14] Their bodies have developed a stronger and longer-lasting immune response via antibodies to the disease.[14] However, those who were treated and cured of Lyme Disease in the early stages may be able to become infected again.[14] This time an individual's body would most likely still have antibodies in your bloodstream, making your infection more difficult to diagnose because.[14] Common detection methods today, as mentioned above, only can detect Borrelia burgdorferi, not an active infection vs an inactive infection.[14] The most likely way to diagnose Lyme Disease again would be the appearance of a bull’s eye.[14]
Unfortunately, it has been found that for those with post-treatment Lyme disease syndrome (PTLDS) no antibiotic treatment is recommended.[13] Previous work has found that some fatigue can be improved with antibiotic treatment for PTLDS individuals.[13] Another study found no improvement with antibiotic treatment in PTLDS individuals.[13] Thus, the overall quality of life does not increase enough that it is recommended to undergo a course of antibiotics for those with PTLDS.[13]
There are a few treatments in the process of discovery. The use of vancomycin is being examined as it has been shown to almost completely destroy Borrelia burgdorferi in vitro during the stationary phase of the spirochetes.[9] Vancomycin seems to target the susceptible cell wall synthesis inhibitors in order to destroy Borrelia burgdorferi.[9] Another group is examining the combination of a few drugs in vitro and currently in mouse models.[9] They are investigating the combination of Doxycycline, which is currently used to treat early Lyme Disease, Cefoperazone, and Daptomycin.[9]
Prevention
One of the best mechanisms for treating Lyme Disease is not getting it to begin with. There are many precautionary steps that can be taken in order to avoid being bitten by an infected tick. Methods of prevention include various factors that help diminish the disease risk.[9] Lyme Disease control methods commonly fall under two categories, the ecological approach, and the human behavior approach.[9] Ecological approaches focus on the vector for the disease itself, the tick, and its transmission.[9] Some suggestions in order to decrease disease risk include reduction of ticks, Lyme Disease hosts (such as white-footed mice or squirrels), and reduction of transmission from hosts to ticks.[9] Any long-term plan to prevent Lyme disease from an ecological approach must take climate change into consideration because as the temperatures increase the locations in which the tick populations and Borrelia burgdorferi can survive will expand.[9] Additional methods of Lyme Disease prevention that have been suggested include, mass culling of white-tailed deer or white-footed mice, as they are both reservoirs for the disease.[9] However, a mass culling has been found to only be effective in isolated populations where Lyme Disease can be completely eliminated.[9] Even if one reservoir is left with the disease in a population, it will spread again.[9] Thus, mass cullings are not seen as a viable option.[9]
The human behavior approach focuses on human behaviors that influence disease risk.[8] Some suggestions include walking closer to the center of paths in order to avoid bushes, wearing long clothes that cover bare skin, wearing bug spray, if possible modifying the environment, checking for ticks after outdoor activities or daily, and if ticks are found on you properly removing them promptly in order to avoid transmission of Borrelia burgdorferi.[8]
If one is bitten by a tick and fits the following criteria then antibiotic treatment may be helpful in taking precautionary measures to avoid Borrelia burgdorferi transmission: 1. Incidence of Borrelia burgdorferi infection, or Lyme Disease, is at least 20% of an individual's area, 2. The tick has been attached for at least 36 hours, 3. Antibiotics can be started within 72 hours of removal of the tick.[8] If individuals do not fit this criterion then antibiotic treatment as a method of prevention is not suggested.[8] Those who have been bitten by a tick should not get tested for Lyme Disease right away and should be monitored for symptoms closely for 30 days.[8] As of today, it is not suggested to get tested right away for Lyme Disease because it takes time for the human body to produce antibodies to the disease.[9] After the 30-day period of time, if experiencing symptoms, individuals may be tested for Lyme Disease as their body would have produced some antibodies by this time.[8]
INSERT IMAGE OF sign from CDC https://www.cdc.gov/ncezid/dvbd/media/lymedisease.html
Vaccines
Two Lyme Disease vaccines have been created and one of which was placed on the market temporarily; however, there are currently no Lyme Disease vaccines on the market. Previous vaccines include LYMErix and ImuLyme.[15] Both of these vaccines utilized the outer membrane protein OspA.[15] The vaccines protect individuals from Lyme Disease by vaccinating against the OspA protein.[15] As a result of this, those vaccinated would then develop anti-OspA antibodies which would bind and neutralize Borrelia burgdorferi that was transmitted from the tick.[15] The Borrelia burgdorferi would not be able to penetrate the skin, thus not infecting an individual.[15]
The first vaccine, LYMErix, was created and placed on the market in December of 1998.[15] It was found that with two doses there was a 49% efficacy rate and with three doses a 76% efficacy rate.[15] The three doses must be given over a one-year period of time, and the vaccine is not considered effective until after all three doses.[15] Thus, people may not be considered safe until after all three doses.[15]
Despite the vaccine being safe and somewhat effective, LYMErix was taken off the market.[15] LYMErix was removed in 2002 from the market and after stage three clinical trials in 1998.[15] LYMErix was removed from the market due to low demand.[15] This was a result of the vaccine only being marketed to people with high-risk jobs, instead of to the general public as precautionary measures.[15] Additionally, Borrelia burgdorferi’s geographical location is limited thus limiting the number of individuals within the market for the vaccine.<[15] Other issues with LYMErix included only having a 76% affection rating, which the FDA deemed as inefficient in preventing Lyme Disease.[15] Additionally, human clinical trials for the vaccine did not include anyone below the age of 15, which is a group that is considered at higher risk for Lyme Disease.[15] Controversy also surrounded the company as the vaccine was suspected to cause arthritis.[15] This claim was dismissed after an investigation by the FDA in 1998.[15] However, the controversies remained present within conversations surrounding LYMErix.[15]
ImuLyme was developed in 1998 however never made it to the market for public use.[16] Two to three doses were necessary for the vaccine to be deemed effective.[15] However, the controversies remained present within conversations surrounding LYMErix.[15] With two doses 68% efficacy was found and with three doses 92% efficacy was found.[15] The efficacy was higher than LYMErix likely due to its difference in composition.[17] ImuLyme has a chemical composition of purified lipoproteins, which serve as important proteins within the disease.[17] The purified proteins did not need to absorb the adjuvant, thus allowing it to be overall more effective.[17]
ImuLyme never made it to the market because the company decided to not produce the vaccine by not pursuing licensure.[17] Their vaccine was set to hit the market at the same time as LYMErix was and the manufacturers of ImuLyme understood that there was not a large enough demand in the market for them both.[17] The profit was seen as too small, thus the vaccine was never produced.[17] Additionally, the vaccine was found to be not as effective in those over the age of 60, which was another age group at high risk of contracting Lyme Disease.<[15]
Potential future vaccines should take multiple things into consideration in order to be deemed effective and avoid previous vaccine flaws. Due to the nature of the outer surface proteins rapidly changing, it is difficult to target Borrelia burgdorferi and destroy it.[15] In order to effectively target Borrelia burgdorferi, a potential vaccine needs to find a protein that is continuously expressed.[15] OspA, outer surface protein A, does just this.[15] Given that it is expressed throughout all stages of the tick during potential transmission, it fits the criteria for a protein to target with a vaccine.[15] This is the same outer surface protein that was targeted in the previous vaccines.[15] While the vaccines worked, they were not as effective as the market would have liked.[15] Thus, future vaccines need to keep in mind these previous vaccine engineering flaws in order to make a new vaccine that is effective.[15] However, the new vaccine would be difficult to properly engineer because OspA is only continuously expressed in the tick midgut.[15] Therefore, the anti-OspA antibodies would need to travel to the midgut in order to destroy the bacteria before traveling to the salary glands of the tick to transmit Borrelia burgdorferi.[15] Additionally, criteria for a future vaccine from a marketing value perspective include both private and public business coordination to develop the vaccine from a public health standpoint and educate individuals that the components of the vaccine are safe.[15]
There are currently multiple vaccines in development. These new vaccines are attempting to address the hardships of the previous vaccines, including the efficacy and genetic diversity of Borrelia species.[9] One vaccine, VLA15, is using six different recombinant OspA proteins in order to target as many spirochetes as possible.[9] VLA15 is the only vaccine currently in human clinical trials.[9] Another vaccine is combining antigens from different stages of infection of Borrelia.[9] This is aimed to target Borrelia that is not killed within the tick midgut or blocked from exiting.[9] Perhaps the most interesting vaccine currently in the works is not specifically an anti-Borrelia vaccine but rather an anti-tick vaccine.[9] An anti-tick vaccine is seen as a precautionary step for many tick-borne illnesses.[9] Ixodes ticks specifically are known to cause 16 human pathogens.[9] With this anti-tick vaccine, individuals could be protected against all 16 pathogens.[9]
Conclusion
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Tilly K, Rosa PA, Stewart PE. Biology of Infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22: 217–234. doi:10.1016/j.idc.2007.12.013
- ↑ Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science (New York, NY). 1982;216: 1317–1319. doi:10.1126/science.7043737
- ↑ 3.0 3.1 3.2 Motaleb MA, Liu J, Wooten RM. Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Current Opinion in Microbiology. 2015;28: 106–113. doi:10.1016/j.mib.2015.09.006
- ↑ Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc Natl Acad Sci U S A. 2000;97: 10899–10904.
- ↑ Zückert WR. Laboratory Maintenance of Borrelia burgdorferi. Current Protocols in Microbiology. 2007;4: 12C.1.1-12C.1.10. doi:10.1002/9780471729259.mc12c01s4
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390: 580–586. doi:10.1038/37551
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46: 515–536. doi:10.1146/annurev-genet-011112-112140
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 9.15 9.16 9.17 9.18 9.19 9.20 9.21 9.22 9.23 9.24 9.25 9.26 9.27 9.28 9.29 9.30 Bobe JR, Jutras BL, Horn EJ, Embers ME, Bailey A, Moritz RL, et al. Recent Progress in Lyme Disease and Remaining Challenges. Frontiers in Medicine. 2021;8. Available: https://www.frontiersin.org/article/10.3389/fmed.2021.666554
- ↑ 10.0 10.1 10.2 CDC. Transmission of Lyme disease | CDC. In: Centers for Disease Control and Prevention [Internet. 29 Jan 2020 [cited 18 Apr 2022]. Available: https://www.cdc.gov/lyme/transmission/index.html]
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 Tortora, Gerard J.; Funke, Berdell R.; Case, Christine L. (2013). Microbiology: An Introduction. United States of America: Pearson Education, Inc. pp. 658–659. ISBN 978-0-321-73360-3
- ↑ 12.0 12.1 12.2 12.3 Skar GL, Simonsen KA. Lyme Disease. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available: http://www.ncbi.nlm.nih.gov/books/NBK431066/
- ↑ 13.0 13.1 13.2 13.3 13.4 NIH. Lyme Disease Antibiotic Treatment Research | NIH: National Institute of Allergy and Infectious Diseases. 2018 [cited 18 Apr 2022. Available: https://www.niaid.nih.gov/diseases-conditions/lyme-disease-antibiotic-treatment-research]
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 15.12 15.13 15.14 15.15 15.16 15.17 15.18 15.19 15.20 15.21 15.22 15.23 15.24 15.25 15.26 15.27 15.28 15.29 15.30 15.31 15.32 Poland GA. Vaccines against Lyme Disease: What Happened and What Lessons Can We Learn? Clinical Infectious Diseases. 2011;52: s253–s258. doi:10.1093/cid/ciq116
- ↑ Nesich H. The History Behind the First Lyme Disease Vaccine – Tick Talk. [cited 18 Apr 2022. Available: https://sites.newpaltz.edu/ticktalk/ottaway-2014-lyme-disease-investigation/history-behind-the-first-lyme-disease-vaccine/]
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 Dattwyler RJ, Gomes-Solecki M. The year that shaped the outcome of the OspA vaccine for human Lyme disease. npj Vaccines. 2022;7: 1–5. doi:10.1038/s41541-022-00429-5
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College