Borrelia burgdorferi and Lyme Disease Pathogenesis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 71: | Line 71: | ||
This idea was first suggested when it was found that <i>B. Burgendorferi<i> was unable to transport iron across its membrane and had very low levels of intracellular iron. It was reasonable to assume then that the bacteria did not rely on iron but some other metal that they had adapted to using. They found that changing the extracellular manganese concentrations affected the expression levels of the protein SodA, responsible for protecting the anaerobic bacteria from oxygen <ref> Troxell, B., H. Xu, and X. Yang. "Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin." Journal of Biological Chemistry. Journal of Biological Chemistry, 12 Apr. 2012. Web. <http://www.jbc.org/content/287/23/19284.long>. </ref>. The expression level was also hindered by the addition of extracellular zinc to the mixture (Figure 5). | This idea was first suggested when it was found that <i>B. Burgendorferi<i> was unable to transport iron across its membrane and had very low levels of intracellular iron. It was reasonable to assume then that the bacteria did not rely on iron but some other metal that they had adapted to using. They found that changing the extracellular manganese concentrations affected the expression levels of the protein SodA, responsible for protecting the anaerobic bacteria from oxygen <ref> Troxell, B., H. Xu, and X. Yang. "Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin." Journal of Biological Chemistry. Journal of Biological Chemistry, 12 Apr. 2012. Web. <http://www.jbc.org/content/287/23/19284.long>. </ref>. The expression level was also hindered by the addition of extracellular zinc to the mixture (Figure 5). Figure 5 shows a western blot of the expression of the protein SodA. The researches exposed the bacteria to either a treatment of manganese (MnCl2) or zinc (ZnSO4) or a combination of the two. Figure 5 shows that greater expression of SodA is only seen when the bacteria is exposed to a zinc solution. | ||
Revision as of 13:38, 12 May 2016
Research Question: Why is the bacterium so good at evading the host's immune response?
Section 1: Overview of Borellia Burgdorferi
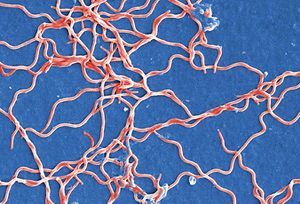
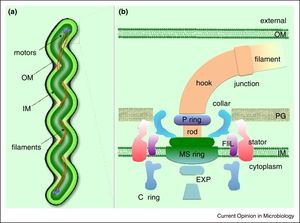
Borellia Burgendorferi is a spirochete bacterium. Like many spirochete species it is very small, with a width of about 0.3 μm and a length of about 5-20 μm. B. Burgendorferi shares the typical spirochete morphology with an outer membrane surrounding an inner membrane. In between these two membranes there are flageller filaments that wrap around the cell and are rotated via a flageller motor to propel the bacterium in a corkscrew motion (Figure 2). It is believed that this corkscrew motion helps the bacterium move through highly viscous environments [1]. The bacterium has a very slow replication rate, which is the one of the reasons it is very hard to identify infection based on blood cell levels [2]. It is also believed that this slow replication time might assist with the bacterium avoiding the host immune response but that will be covered below.
Section 2: Lyme Disease Overview
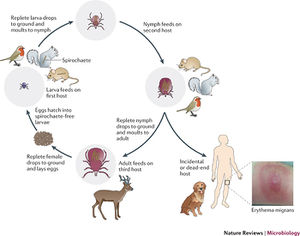
Lyme disease was first noticed by Doctor Alan Steere in 1977, when he noticed that persons were being infected by an unknown pathogen producing the same symptoms in the same geographical region and during the same times of the year. It was finally discovered that the infection was due to spirochete bacteria found in the midgut of ticks local to the area. The infection was found to create a rash and the bacteria was named Borellia Burgendorferi. Ticks were found to have taken in the bacteria while still larvae and feeding on rodent carriers. Once these ticks grow up into their nymph and adult stages they feed on all species of larger hosts, thus transmitting the bacteria on. They also will feed on more rodents, some who aren’t infected, hence perpetuating the issue (Figure 3). Once a tick bites a human host B. Burgendorferi bacteria migrate to the salivary glands of the ticks and into the blood stream of the new host. The bacteria will then travel through the blood stream of the host, eventually settling in various tissues and causing an inflammatory response and damage [3].
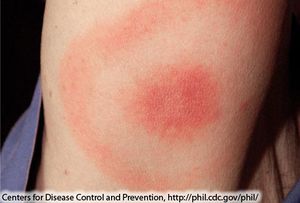
B. Burgendorferi does not actually contain any virulence factors and the damage done is due to necessary steps for the bacterium to replicate. The damage done is actually due to a stimulation of the immune system, which attacks, damaging the tissue around the bacteria. This response by the immune system is actually what causes the symptoms of Lyme disease, and is the reason why the symptoms can vary so much due to the fact that the areas damaged by the immune system will create varying symptoms [4]. For example if the ganglia and glial cells are damaged then the host will suffer from neurological symptoms like tremors, arthritis, or seizures, and pain [5]. A local immune response at the site of infection can cause a erythema migrans (EM) rash (Figure 4) [6].
Section 3: B. Burgendorferi use of magnesium to avoid immune response
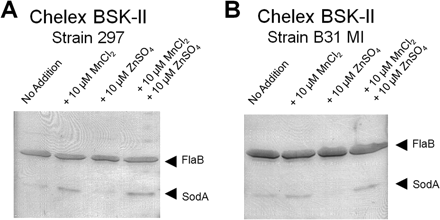
One of the host’s immune responses to an infection is to limit the production and concentration of iron in the body. This is due to many cells, including pathogenic bacteria, requiring iron for the production of enzymes essential to growth. By limiting the iron levels, and the ability for bacteria to grow, the body can possibly get the upper hand on fighting the infection. This is done by the release of the hormone hepciden by the liver, which lowers iron levels. This makes the person anemic which is why people feel tired and weak when they are sick [7]. Researchers at the Indiana University School of Medicine found that B. Borgendorferi has eliminated this issue by substituting manganese for iron in making the enzyme.
This idea was first suggested when it was found that B. Burgendorferi was unable to transport iron across its membrane and had very low levels of intracellular iron. It was reasonable to assume then that the bacteria did not rely on iron but some other metal that they had adapted to using. They found that changing the extracellular manganese concentrations affected the expression levels of the protein SodA, responsible for protecting the anaerobic bacteria from oxygen [8]. The expression level was also hindered by the addition of extracellular zinc to the mixture (Figure 5). Figure 5 shows a western blot of the expression of the protein SodA. The researches exposed the bacteria to either a treatment of manganese (MnCl2) or zinc (ZnSO4) or a combination of the two. Figure 5 shows that greater expression of SodA is only seen when the bacteria is exposed to a zinc solution.
The authors found some more interesting discoveries about these pathways. They found that when growth in an environment of streptonigrin, an antibiotic, the bacterial growth was highly limited. The effects of streptonigrin were lessoned when manganese was added to the environment. It is believed that the binding of manganese to the SodA protein limits the effect of the antibiotic.
With all of these results in mind it points to a possible treatment for Lyme disease that could be researched into. Although the use of manganese hides the bacteria from the host’s immune response of limiting iron availability, it also creates a specific place to attack the bacteria. If manganese can be removed or limited to the bacteria it could slow or even stop growth. Further it was found that the addition of zinc would possibly increase the effects of antibiotics on the bacterial infection. Further research would have to be done but it could be a promising start for Lyme disease treatment.
Section 4: Tick saliva immunosuppressant
The evasion of the host’s immune response can be observed in B. Burgendorferi infection the moment the bacteria enters the bloodstream via the bite of a tick. It was discovered by a research group at the University of Massachusetts, that tick saliva contains the salivary protein Salp15. This protein, they hypothesized, would hinder the host immune response via the deactivation of T-cells [9].
T-cells are vital to the response of the immune system in the human body. T-cells will circulate throughout the human body searching for infected cells and pathogens. There are two types of T-cells, known as killer T-cells and helper T-cells. The killer T-cells will detect a foreign pathogen or an infected cell based on the peptides on the surface of the cells and engulf and destroy these cells. Infected cells will display the pathogen’s peptide on its cell surface. Helper T-cells will also identify an infected cell or pathogen but will help by recruiting and signaling macrophages and killer T-cells to multiply and localize to the infection area [10].
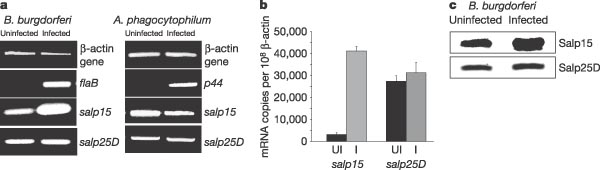
One receptor protein CD4, a co-receptor protein, is found to be very important in T-cell activation, as the binding of the protein to MHC complex is responsible for the activation of T-cells [11]. The researchers found, in a previous study conducted by them, that Salp15 binds to the CD4 complex. Figure 6 to the right shows the results of one of their experiments. They first found that CD4 deficient T-cells had far less immunosuppressive activity. This is shown by the open bars (CD4+ T-cells) compared to the filled bars (CD4-) cells. They also found that T-cells grown in Salp15 environment also had decreased immunosuppressive activity. They concluded that the binding of Salp15 to the CD4 complex on T-cells prevents them inducing the activation pathways to incite an immune response.
For a tick the Salp15 protein is important in suppressing the immune response since the tick needs to feed for a few days on the host’s blood in order to get the nutrients it needs to survive. If the host’s immune system works at the site of attachment the tick risks being attacked and having to detach before it dies. This same local immune response suppression could advantageous for the B. Burgendorferi bacteria as it gives the bacteria the time to invade the host’s tissues without being detected so it can multiply. In 2005, Ramamoorthi et. al found that both in vitro and in vivo that B. Burgendorferi bound to the Salp15 protein [11]. They also found that Salp15 is upregulated in ticks infected with B. Burgendorferi suggesting that the bacteria might physically up regulate the expression of the protein it needs to evade the human host immune system in the tick (Figure 6). In the figure they compare the regulation of the Salp15 protein in B. Burgendorferi infected ticks to the regulation of Salp15 in Anaplasma phagocytophilum infected mice. A. phagocytophilum is another tick vectored bacteria that can be transferred to humans. As you can see from Figure 6, ticks infected with A. phagocytophilum did not have greater expression of the Salp15 protein, indicating it is not necessary for transmission of the bacteria to another host.
The findings of these results have larger implications besides merely understanding the route of B. Burgendorferi infection. An overactive immune system, especially overactive and misguided T-cells cause a variety of autoimmune diseases. The failure of T-cells to correctly identify the host cells from tissues can cause the immune system to attack the host cells. For example in Crohn’s disease the immune system attacks the lining of the gut, creating inflammation, pain, and digestive issues [10]. The suppressive results of Salp15 may lead to a possible therapy for autoimmune diseases, many of which have no definitive treatment [8]. Obviously there are some downsides to suppressing T-cell efficiency and further the efficiency of the host’s immune system but the results of the study bring up a very interesting mode of future research.
Section 5: B. Burgendorferi escaping the blood stream
A study by Moriarty et al. in 2008 sought to understand the mechanism of interaction between B. Burgendorferi and the vasculature of the infected host. Due to the small size of the spirochete bacteria it has previously been difficult to observe the interaction of the bacteria with the host. It was known that B. Burgendorferi could be found in many different tissues throughout the body but it was unknown how they got there or why the bacteria would decide to locate themselves in that specific tissue. Obviously they would circulate through the lymph or cardiovascular systems until they reached their destinations and imbedded in the tissues but the purpose of this was unknown.
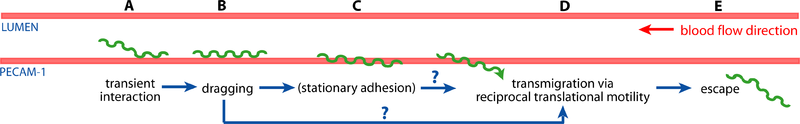
The researchers first had to create a method to visualize the bacteria in real time. Again due to their size they couldn’t use standard light microscopy. They ended up creating a fluorescent strain of B. Burgendorferi that expressed the green fluorescent protein (GFP). This allowed them then to do real time fluorescent microscopy of the interaction between the fluorescent bacteria and the vasculature of a living mouse host.
The researchers found that the bacteria would interact with the vasculature in a few ways, outlined in Figure 7. As observed it was possible for the bacterium to either partially invade the intercellular junction of the epithelial lumen of the blood vessel or completely escape the blood vessel. This proved that the bacteria was able to escape the blood stream and thus possibly evade the host’s immune system, such that at a later point the same bacteria can reinfect the blood stream of the host [12].
Section 6: Borrelia burgendorferi resistance to antibiotics
Despite treatment with antibiotics there are a persistence of symptoms and even reoccurrence of infection. Thus it can be concluded that B. Burgendorferi has some mechanism to resist the effect of antibiotics. In 2010 Barthold et al. showed the resistance of B. Burgendorferi to tigecycline. Their results showed that, compared to a saline control, even though treatments of either high or low doses of tigecyline lowered the number of B. Burgendorferi bacterial markers in the tissues of the infected mice, the antibiotic was unable to fully eradicate the bacteria from the host [13]. This would normally not be an issue as the host’s immune system would eventually clean up the remaining bacteria but as was previously explained, the host’s immune system can not often find the bacteria and destroy it. It was concluded by the Barhold et al. that the bacteria most likely goes into a state of minimal growth and division while still remaining viable, such that when the environment is more suitable (ie. the antibiotic is gone) the bacteria can begin dividing and growing again. This could be the mechanism of reinfection in persons infected with Lyme disease despite antibiotic treatment.
Section 7: Borrelia burgendorferi Outer Surface Proteins and possible vaccine
B. Burgendorferi has a relatively small genome, however can greatly vary the expression of that genome. This is another one of the reasons that the immune system has trouble eliminating the infection. Even if the immune system finds and creates antibodies to one of the B. Burgendorferi's outer surface proteins (Osp), the bacteria’s ability to quickly change the expression of that protein means it can eliminate the effectiveness of the antibody [14]. This is also one reason why it would be very difficult to create a vaccine that would incite antibody production by the human body to combat a possible infection by B. Burgendorferi. The challenge to create a vaccine was to find a protein that is continually expressed and thus be a target for antigens. One outer surface protein, OspA, was expressed continuously while in the tick midgut. It is believed that it is responsible for the migration of the bacteria from the midgut to the salivary glands when a tick is feeding. Once the bacteria enter the body it no longer has use for the protein and it is down regulated. Thus a vaccine to this protein could potentially destroy the bacteria. Due to the protein only being expressed while in the midgut of the tick, the host anti-OspA antibodies would have to travel into the tick and destroy the bacteria before it traveled to the salivary glands [15].
Two vaccines using OspA were actually produced and put to market, however both of them were taken off the market four year later. The first, LYMErix, was released in 1998 and showed 76% efficacy against Lyme disease with three doses. The second, Imulyme, produced after LYMErix was found to have a 92% efficacy against Lyme disease. The reason for the discontinuation of the vaccines had more to do with financial issues rather than the effectiveness or safety of the vaccine. The first issue was with the fairly small demand for the vaccine. This had to do with the limited geographical locations of ticks containing the bacteria (limited to the east coast in the United States), and that the vaccine was marketed only for persons in high risk occupations rather than just as a general precaution. Secondly there were unfounded concerns on the safety of the vaccine. There were reports of autoimmune arthritis side effects of the vaccine and some argued that the long term effects were still unknown. An FDA safety panel was created in 2001 to investigate those claims and found that the incidence of autoimmune arthritis was no higher in vaccinated persons then in the background rate in un-vaccinated persons. Despite this there were anti-vaccine groups that continued to challenge the safety of the vaccine and launched lawsuits to ban it. Imulyme quickly pulled its vaccine from the market because of the limited demand [16]. Shortly after LYMErix pulled its vaccine due to mounting lawsuits and a decreasing demand for the vaccine. Since then no further vaccine against OspA has been released.
Section 8: Current Research
Much of the current research into Lyme disease and B. Burgendorferi centers on better techniques for early detection in infected individuals. Current testing for Lyme disease test for the presence of common antibodies to B. Burgendorferi, which could indicate an infection. There are two problems with this mode of diagnosis. In some cases, especially in the early stages of infection, there might not be enough antibodies to constitute a positive antibody test result. Secondly the presence of the antibodies does not necessarily conclude an active infection and those individuals might already have been exposed and overcome the bacterial infection. This is an issue because in those cases you would be giving people antibiotics that don’t need them, which could increase the possibility of antibiotic resistance, a major medical issue in the world [17].
There is also debate as to the origin of what is known as chronic Lyme disease. This issue results when, despite negative testing after treatment, persons still notice the symptoms of Lyme disease including fatigue and chronic pain. The reasoning behind this is under some contention. A study done by the Radbound University Health Center in the Netherlands found that an additional 12 weeks of antibiotic treatment did not lessen the symptoms of patients that had been infected with Lyme disease, even those who tested negative for an infection. This indicates that damage caused by the infection, especially to the nervous system, could manifest itself as “chronic Lyme disease”. Some doctors contest this finding, as they believe that the only way the symptoms could continue would be because the infection has not been wiped out. They assert that the reason for the negative antibody test is due to the last of sensitivity or accuracy of the test and thus the results are invalid. Although there is no definitive proof behind this, the conclusion at least appears reasonable, especially because of the bacteria’s ability to evade the host’s immune system [18].
References
- ↑ Motaleb, A., J. Liu, and R. Wooten. "Spirochetal Motility and Chemotaxis in the Natural Enzootic Cycle and Development of Lyme Disease." Science Direct. N.p., 2 Nov. 2015. Web. <http://www.sciencedirect.com/science/article/pii/S1369527415001368>.
- ↑ "What Causes Lyme Disease?" Bay Area Lyme Foundation. N.p., 2012. Web. 25 Apr. 2016. <http://www.bayarealyme.org/about-lyme/what-causes-lyme-disease/borrelia-burgdorferi/>.
- ↑ Tilly, Kit, Patricia A. Rosa, and Philip E. Stewart. "Biology of Infection with Borrelia Burgdorferi." Infectious Disease Clinics of North America. U.S. National Library of Medicine, 22 June 2007. Web. 25 Apr. 2016. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2440571/#R91>.
- ↑ Tilly, Kit, Patricia A. Rosa, and Philip E. Stewart. "Biology of Infection with Borrelia Burgdorferi." Infectious Disease Clinics of North America. U.S. National Library of Medicine, 22 June 2007. Web. 25 Apr. 2016. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2440571/#R91>.
- ↑ Ramesh, Geeta, Lenay Gould, Fiona Inglis, John England, and Mario Philipp. "The Lyme Disease Spirochete Borrelia Burgdorferi Induces Inflammation and Apoptosis in Cells from Dorsal Root Ganglia." Journal of Neuroinflammation. Journal of Neuroinflammation, July 2013. Web. 25 Apr. 2016. <http://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-10-88>.
- ↑ "Signs and Symptoms of Untreated Lyme Disease." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 17 Aug. 2015. Web. 25 Apr. 2016. <http://www.cdc.gov/lyme/signs_symptoms/index.html>.
- ↑ "Scientists Reveal Quirky Feature of Lyme Disease Bacteria." PHYS.org. N.p., 21 Mar. 2013. Web. 25 Apr. 2016. <http://phys.org/news/2013-03-scientists-reveal-quirky-feature-lyme.html>.
- ↑ Troxell, B., H. Xu, and X. Yang. "Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin." Journal of Biological Chemistry. Journal of Biological Chemistry, 12 Apr. 2012. Web. <http://www.jbc.org/content/287/23/19284.long>.
- ↑ Juncadella, I., R. Garg, S. Ananthnarayanan, C. Yango, and J. Anguita. "T-cell Signaling Pathways Inhibited by the Tick Saliva Immunosuppressor, Salp15." Wiley Online Library. FEMS Immunology & Medical Microbiology, Apr. 2007. Web. <http://onlinelibrary.wiley.com/doi/10.1111/j.1574-695X.2007.00223.x/full>.
- ↑ "Beginners Guide to T Cells." T-cell Modulation Group. N.p., 2009. Web. 25 Apr. 2016. <http://www.tcells.org/beginners/tcells/>.
- ↑ Juncadella, I., R. Garg, S. Ananthnarayanan, C. Yango, and J. Anguita. "T-cell Signaling Pathways Inhibited by the Tick Saliva Immunosuppressor, Salp15." Wiley Online Library. FEMS Immunology & Medical Microbiology, Apr. 2007. Web. <http://onlinelibrary.wiley.com/doi/10.1111/j.1574-695X.2007.00223.x/full>.
- ↑ Moriarty, T., and M. Norman. "Real-Time High Resolution 3D Imaging of the Lyme Disease Spirochete Adhering to and Escaping from the Vasculature of a Living Host." PLOS. PLOS ONE, 20 June 2008. Web. 25 Apr. 2016. <http://journals.plos.org/plospathogens/article?id=10.1371%2Fjournal.ppat.1000090>.
- ↑ Barthold, Stephen W., Emir Hodzic, Denise M. Imai, Sunlian Feng, Xiaohua Yang, and Benjamin J. Luft. "Ineffectiveness of Tigecycline against Persistent Borrelia Burgdorferi." Antimicrobial Agents and Chemotherapy. American Society for Microbiology (ASM), Feb. 2010. Web. 25 Apr. 2016. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2812145/>.
- ↑ Tilly, Kit, Patricia A. Rosa, and Philip E. Stewart. "Biology of Infection with Borrelia Burgdorferi." Infectious Disease Clinics of North America. U.S. National Library of Medicine, 22 June 2007. Web. 25 Apr. 2016. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2440571/#R91>.
- ↑ Poland, G. "Vaccines against Lyme Disease: What Happened and What Lessons Can We Learn?" Clinical Infectious Disease. Clinical Infectious Disease, n.d. Web. <http://cid.oxfordjournals.org/content/52/suppl_3/s253.full>.
- ↑ Poland, G. "Vaccines against Lyme Disease: What Happened and What Lessons Can We Learn?" Clinical Infectious Disease. Clinical Infectious Disease, n.d. Web. <http://cid.oxfordjournals.org/content/52/suppl_3/s253.full>.
- ↑ Medical University of Vienna. "New test for early detection of Lyme disease developed." ScienceDaily. ScienceDaily, 25 April 2016. <www.sciencedaily.com/releases/2016/04/160425095342.htm>.
- ↑ Park, Alice. "Treating Chronic Lyme With Long-Term Drugs Doesn't Help."Time. Time, 1 Apr. 2016. Web. 25 Apr. 2016. <http://time.com/4276976/chronic-lyme-treatment-antibiotics/>.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
