Chlorobium FMO antenna complex characterisation
Discovery of the Fenna-Matthews-Olson Protein Membrane Orientation in Chlorobaculum tepidum
A team of researchers at Washington University in St. Louis, lead by Robert Blankenship, has pioneered a new method of discovering protein orientation in living systems (1). By combining chemical labeling with mass spectroscopy, these scientists brought forth knowledge of the structure/function relationship of the Fenna-Matthews-Olson (FMO) protein in Chlorobaculum tepidum. C. tepidum is a member of the Chlorobium bacteria and are closely related, yet distinct from, the Bacteroides phylum (2).

Characteristics of Green Sulfur Bacteria
In the absence of light, life on earth would not exist. Biological photosynthesis is among the most important reactions on planet earth that allows life to thrive. It was long thought that life was restricted primarily to photic zones where photosynthetic primary producers could access the light needed to drive their metabolism. This view was challenged with the discovery of green sulfur bacteria that grow deep in the ocean floor. Green sulfur bacteria are photosynthetic microbes that are capable of thriving in ecological regions that have extremely low levels of solar radiation. It has been proposed that certain types of green sulfur bacteria use what is known as Geothermal Light to supplement their chemotrophic metabolism. The emission of photons from geothermal vents could have provided a selective advantage to chemotrophic ancestors of modern day green sulfur bacteria that utilized light sensing systems to perform phototaxis towards sulfur-rich geothermal vents (3). A new microbial world at the bottom of the ocean adds to our knowledge of the incredible biological diversity on our planet and sheds light on a new ecological system.
On Earth, the discovery of a photosynthetic species capable of using geothermal radiation, rather than solar sources, helps support the hypothesis that life exists on other planets devoid of star-light (10). In addition to providing supporting evidence for extraterrestrial life, it is speculated by some that life on Earth originated in conditions similar to those displayed in the vicinity surrounding deep sea hydrothermal vents (11).
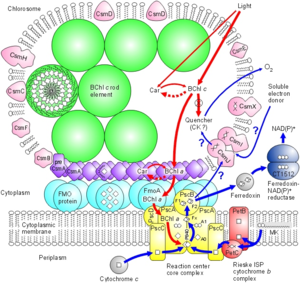
In green sulfur bacteria, the harvesting of light is in part carried out by the Fenna-Matthews-Olson protein (FMO). Based on the current literature, the FMO protein is thought to be unique to the green sulfur bacteria (4). The chlorobiaceae contain Fe-S type reaction centers that share an ancestor with oxygenic photosynthetic organisms that utilize photosystem I (4).
Introduction to the FMO protein
In C. tepidum, the Fenna-Matthews-Olson protein is part of a light-harvesting complex that consists of three major components: 1. Chlorosome 2. Aforementioned FMO protein, and 3. Reaction center. This system captures electromagnetic radiation from the sun and forms a funnel-like system that directs energy transfer (3). The role of the FMO protein is analogous to that of a wire connecting two electrodes. That is to say that the FMO protein provides the transfer of energy from the chlorosome to the reaction center.
The research of Wen, Zhang, Gross et al. (2008) is important in understanding not only the structure of the BChl a protein, but its orientation within the bacterial cell membrane (9).
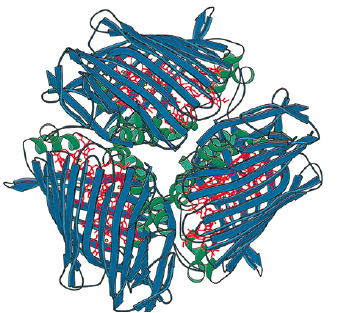
The Fenna-Matthews-Olson protein complex has been identified in all surveyed green sulfur bacteria to date (4). During the late 1970’s, the FMO complex was first described in the organism Prosthecochloris aestuarii (5, 6). The FMO protein complex contains pigments that molecular biologists refer to as bacteriochlorophyll proteins. In the FMO complex, seven bacteriochlorophyll proteins are present (BChl a). The FMO complex has a hetero-trimeric structure that has been resolved at a maximum resolution of 1.9 angstroms (7). With this high resolution, knowledge of the structure has provided a background for the optical analysis of the protein. However, as is the case with many advancing work in science, the theoretical framework for this optical analysis has been disputed by other researchers (8). More recent work has been done to elucidate both the structure of the FMO complex and its orientation in relation to the cell membrane, the cytoplasmic membrane, and the reaction center (4). Each of the three monomers in the FMO complex have a mass of 40 kDa and form 3-fold symmetry (4). Together, these three monomers form C-3 symmetry and have an axis that is thought to be perpendicular to the cellular membrane. Each of the three monomers contains seven of the BChl a molecules in addition to another eighth pigment (12, 13). The BChl a protein plays a critical role in transferring light energy to the reaction complex. BChl a molecules function through excitons of individually excited molecules (4, 6). The strength of the interactions of these excitons depends on the orientation of the BChl a proteins, as well as the distance between them (4, 6). Recently discovered quantum effects of within the FMO complex are thought to provide more efficient energy transfer as the light energy travels from the cytoplasmic membrane to the reaction center (14, 15, 16). The researchers have assigned numbers to the individuals BChl a monomers to help with communication aimed towards verifying the orientation of the FMO complex. For example, BChl a #3 (named so for historical reasons) is thought to have the lowest site energy. This suggests that the FMO complex is oriented such that BChl a#3 is opposite of the reaction center and facing towards the cytoplasmic membrane. Converse to the evidence in support of this theory are the results of research that examined the structure and properties of the isolated protein. Investigations of the hydrophobicity of the protein suggest that BChl a# 1 is facing the the cytoplasmic membrane. This orientation model is the exact opposite of that suggested by Blankenship et al. (2008).
Methods of studying the orientation of the FMO complex have included linear dichroism (17) and 3D reconstructions made possible by STEM imaging (18). These two methods have been useful in suggesting that the FMO disc flat with its C3 axis of symmetry perpendicular to the cytoplasmic membrane, but they are not insightful with regards to which side of the FMO disc actually contacts or faces the cytoplasmic membrane. Other details of the overall antenna complex remain unclear, as well (4). Within the chlorosome, the interaction of the Csma protein with the FMO disc is not clear but there is evidence to suggest that Csma and the FMO trimer are in direct contact and that, perhaps, the FMO complex is partially submersed within a layer of Csma proteins (19). Zhou, Blankenship et al. (1997) took asked whether or not the complex is embedded in the cellular membrane by examining the assemblage of amino acids in the trimer (4). They found that the top surface of the BChl a trimer has mostly hydrophilic amino acid residues and that the bottom surface has relatively more hydrophobic residues. According to their analysis, up to forty-percent of the BChl a trimer may be embedded in the membrane. Furthermore, this evidence is in agreement with the linear dichroism studies conducted on the BChl a protein isolated from P. aestuarii and C. tepidum (20).
Chlorobaculum tepidum Fenna-Matthews-Olson (FMO) Antenna Protein
In C. tepidum, the Fenna-Matthews-Olson protein is part of a light-harvesting complex that consists of three major components: 1. Chlorosome 2. Aforementioned FMO protein, and 3. Reaction center. This system captures electromagnetic radiation from the sun and forms a funnel-like system that directs energy transfer (3). The role of the FMO protein is analogous to that of a wire connecting two electrodes. That is to say that the FMO protein provides the transfer of energy from the chlorosome to the reaction center.
The research of Wen, Zhang, Gross et al. (2008) is important in understanding not only the structure of the BChl a protein, but its orientation within the bacterial cell membrane (9).

Include some current research in each topic, with at least one figure showing data
Relating the Light Harvesting Systems of Other Species to the BChl a Protein Structure in Green Sulfur Bacteria
Include some current research in each topic, with at least one figure showing data.
[edit] Discovering the Shape
Overall paper length should be 3,000 words, with at least 3 figures.
References
(1)Washington University in St. Louis. "'Taco Shell' Protein: Orientation Of Antenna Protein In Photosynthetic Bacteria Described." ScienceDaily 9 April 2009. 12 April 2009 <http://www.sciencedaily.com¬ /releases/2009/04/090402171438.htm>.
(2)D.A. Bryant & N.-U. Frigaard (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. 14 (11): 488. doi:10.1016/j.tim.2006.09.001
(3)Beatty, J.T.; Overmann, J.; Lince, M.T.; Mansket, A.K.; Lang, A.S.; Blankenship, R.E.; Van Dover, C.L.; Martinson, T.A.; Plumley, F.G. “ An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent”. PNAS June 28, 2005 vol. 102 no. 26 9306-9310
(4)Li YF, Zhou W, Blankenship RE, Allen JP (1997) Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol 271:456–471.
(5)Olson, J. M. (1978). Bacteriochlorophyll a-proteins from green bacteria. In The Photosynthetic Bacteria (Clayton, R. K. & Sistrom, W. R., eds), pp. 161± 178, Plenum Press, New York
(6)Olson, J. M., Ke, B. & Thompson, K. H. (1976). Exciton interaction among chlorophyll molecules in bacteriochlorophyll a proteins and bacteriochlorophyll a reaction center complexes from green bacteria. Biochem. Biophys. Acta, 430, 524±537.
(7)Tronrud, D. E. & Matthews, B. W. (1993). Refinement of the structure of a water-soluble antenna complex from green photosynthetic bacteria by incorporation of the chemically determined amino acid sequence. In The Photosynthetic Reaction Center (Norris, J. & Deisenhofer, J., eds), vol. 1, pp. 13±21, Academic Press, New York.
(8)Gülen, D. (1996). Interpretation of the excited-state structure of the Fenna-Matthews-Olson pigment protein complex of Prosthecochloris aestuarii based on the simultaneous simulation of the 4K absorption, linear dichroism, and singlet-triplet absorption difference spectra: a possible excitonic explanation?. J. Phys. Chem. 100, 17683±17689.
(9)Wen, J.; Zhang, H.; Gross, M.L.; Blankenship,, R.E. (2008) Membrane orientation of the FMO antenna protein from Chlorobaculum tepidum as determined by mass spectrometry-based footprinting. PNAS. (www.pnas.org_cgi_doi_10.1073_pnas.0901691106)
10)Chyba, C. F. & Hand, K. P. (2001) Science 292, 2026–2027
11)Martin, W. & Russell, M. J. (2003) Philos. Trans. R. Soc. London B 358, 59–85.
12)Ben-Shem A, Frolow F, Nelson N (2004) Evolution of Photosystem 1—from symmetry through pseudosymmetry to asymmetry. FEBS Lett 564:274–280.
13)Tronrud DE, Wen JZ, Gay L, Blankenship RE (2009) The structural difference in absorbance spectra for the FMO protein from green sulfur bacteria (submitted).
14)Read EL, et al. (2008) Visualization of excitonic structure in the Fenna-Matthews-Olson photosynthetic complex by polarization-dependent two-dimensional electronic spectroscopy. Biophys J 95:847–856.
15)Mohseni M, Rebentrost P, Lloyd S, Aspuru-Guzik A (2008) Environment-assisted quantum walks in energy transfer of photosynthetic complexes. Quantum Physics arXiv:0805.2741v1.
16)Müh F, et al. (2007) _-Helices direct excitation energy flow in the Fenna-Matthews-Olson protein. Proc Natl Acad Sci USA 104:16862–16867.
17)Melkozernov AN, Olson JM, Li YF, Allen JP, Blankenship RE (1998) Orientation and excitonic interactions of the Fenna-Matthews-Olson Protein in membranes of the green sulfur bacterium Chlorobium tepidum. Photosynth Res 56:315–328.
18)Re´migy HW, et al. (1999) The reaction center complex from the green sulfur bacterium Chlorobium tepidum: A structural analysis by scanning transmission electron microscopy. J Mol Biol 290:851–858.
19) Li H, Frigaard NU, Bryant DA (2006) Molecular contacts for chlorosome envelope proteins revealed by cross-linking studies with chlorosomes from Chlorobium tepidum. Biochemistry 45:9095–9103.
20) Swarthoff, T., de Grooth, B. G., Meiburg, R. F., Rijgersberg, C. P. & Amesz, J. (1980). Orientation of pigments and pigment-protein complexes in the green photosynthetic bacterium Prosthecochloris aestuarii. Biochim. Biophys. Acta, 593, 51±59.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.