Clostridium perfringens Bacteria and Food Illness
By Paula Cancelas Calvo
Introduction
Food-borne illness occurs when one consumes contaminated foods or beverages. This type of illness has been around humans for as long as we have been humans, and the same pathogens not only affects humans but also animals. In humans, "The Centers for Disease Control and Prevention (CDC) estimate that 76 million foodborne illnesses, including 325,000 hospitalizations and 5,000 deaths, occur in the United States each year" [1]. There are many different types of pathogens that can cause food-borne illness, some less serious than others, nevertheless, they are still serious. Many of these bacteria can lead to serious gastrointestinal complications. Clostridium perfringens is one of those bacteria that is known for food related illness. This gram-positive, nonmotile, anaerobic spore producing bacteria is one of the most common and important human and animal pathogens that can cause an array of diseases [2]. In addition, this pathogen is among the most widespread bacteria, with a ubiquitous environmental distribution in soil, sewage, food, fecal, and in normal intestinal gut microbiota of both humans and animals[2].
In regards to food related illness, Clostridium perfringens is one of the most common causes and the CDC estimates that 1 million illnesses are caused by these food poisoning bacteria[3].. Symptoms associated with consumption of contaminated foods are most often diarrhea and stomach cramps and usually appear 6 to 24 hours after consumption of contaminated food, and this is not transmissible directly from one person to another[3]. This type of food poisoning leaves you in pain and dehydrated. In most, antibiotics are not necessary for treatment and as long as fluids are restored there should be no problems. Although these symptoms are considered to be short and temporary, as mentioned before, C. perfringens can lead to serious gastrointestinal infections such as traumatic gas gangrene, necrotic enteritis of both human and neonatal animal species, enterotoxemia of sheep and goats, and among others, all which can lead to death if left untreated[2]. Not only that, but in agricultural circumstances, it can decimate farm output which can affect a country’s economy and consumers if a serious outbreak were to occur. “According to the United States Department of Agriculture (USDA), foodborne illness costs the US economy $10-83 billion per year”[1].
The reason why C. perfringens infections are of concern is because of how common the bacterium is and how it is the major cause of food-borne illness. The toxins that the bacteria produces are believed to be what damages the host, however, there are many different kinds and it unclear why so many kinds are produced. In addition, with the overprescription and improper disposal of antibiotic medication, through mobile genetic elements or horizontal gene transfer, drug resistance is becoming more prevalent in all sorts of bacterial species including C. perfringens. This antibiotic resistance also affects the gut microbiome in both animals and humans. All of these pose as concern as food-borne illness is a major public health threat, and to better understand the major cause of said illness will improve treatment and prevention methods.
Clostridium perfringens Toxin

Most bacterial infections are caused by potent toxic proteins. The many diseases occur as a result of the bacterium’s toxins it produces, many of which are extracellular [4]. There are >16 toxins that can cause histotoxic and intestinal infections, and it is unclear as to why there are so many different kinds when many have the same target: host cell plasma membranes[2]. Each toxin is produced depending on the type of C. perfringens and there are 5 types: A, B, C, D, and E[2].In figure 1, the structure of different types of C. perfringens toxins are shown.
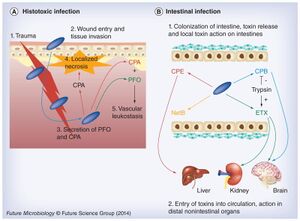
Histotoxic clostridial infection is a type of pathogenesis that involves the growth of Clostridium in the tissues and extensive tissue destruction, which is a result done by the extracellular toxins. Histotoxic infection includes gas gangrene or myonecrosis. It has been established that C. perfringens uses chromosomally encoded alpha toxin (alpha-phospholipase C) and perfringolysin O (a pore-forming toxin) in histotoxic (poisonous to tissue) infections [2]. On the other hand, intestinal disease occurs by employing toxins encoded by mobile genetic elements, including C. perfringens enterotoxin, necrotic enteritis toxin B-like, epsilon toxin and beta toxin[2].
After testing to see the effects specific toxins have on the intestinal walls, some toxins (CPA and PFO) are specifically used for histotoxic infection (fig. 2. A). During histotoxic infection, these toxins are able to interfere with the host’s immune response which causes localized necrosis to facilitate the growth of the pathogen. In addition, the toxins allow for host cells leakage and lysis which further benefits pathogen growth by providing nutrients[2]. This combination leads to cell death and eventually the death of the host, killed cells only improve C. perfringens multiplication.There is also evidence for pore-forming toxins originating in the intestine (fig. 2. B), however, it is still not understood why the pathogen produces so many toxins that are able to perform the same or similarly. One explanation could be that the purpose of producing many kinds of toxins allows for the pathogen to cause various diseases under an array of intestinal conditions. By understanding these toxins better, it will allow for ways to type these different types of Clostridium species, not restricted to C. perfringens.
Drug Resistance
Antibiotic resistance is serious matter that is affecting a wide variety of bacterial species. Because of this it is important for the cattle and animals that farmers grow do not become infected with C. perfringens or any other bacteria. However, antibiotic resistance is spreading through horizontal transfer methods such as mobile genetic elements, which can cross between different species. Therefore, many bacteria species can develop antibiotic resistance, if given the chance. This can pose a problem for the farm and food industry with animals. Since C. perfringens is spread through contaminated meat or fecal matter, for most farm animals, if there is one infected, it will eventually spread to the whole herd. If an animal is infected with a bacteria that can’t be treated due to antibiotic resistance, it will have to be slaughtered. Not only is this costly, but also potentially dangerous if the contaminated meat is consumed.
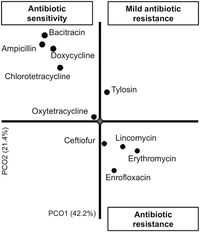
Antibiotic resistance is becoming more common and it too is influencing C. perfringens. Antibiotic-associated diarrhea has been found in approximately 5-15% of all cases, which then causes 5-40% of all patients receiving antibiotic therapy[2]. However, from figure 3, we can see that there are a few antibiotics that C. perfringens is resistant against, such as erythromycin, lincomycin, enrofloxacin,and ceftiofur, but most isolates display antibiotic sensitivity. This means that those tested antibiotics have a greater chance of working on C. perfringens infections, at least in 2016.
The Gut Microbiota
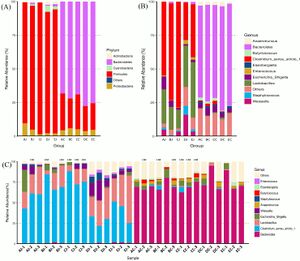
The gut is home to a wide array of microorganisms species, including bacteria and archaea. The population of these bacteria can change as a result of the environmental conditions, in this case the gut. In the case of C. perfringens infection, dysbiosis is a possible occurrence. There is a disease caused by this pathogen called necrotic enteritis (NE) and this is an economically devastating disease that is found commonly in poultry. Not only does it cause dysbiosis, but also lesions[7][6]. The microbiota of the gut vary depending on the region of the intestines which is why figure 4A,4B, and 4C show different proportions of both phyla and genera.
Prevention
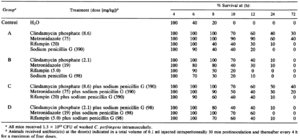
When it comes to potential food contamination, the best way to prevent C. perfringens infection before introduction is to cook food at safe temperatures, especially beef and poultry, and once that food is cooked keep it above 140℉ or below 40℉ [3]. If an individual is already infected and symptoms persist or treatment is necessary, there are many types of medications that can improve cell survival against C. perfringens attack. Of those treatments, when compared to the control (water), cell survival improves vastly after 6 hours (table 2).
Conclusion
Clostridium perfringens is a pathogen that needs to be better understood as they are influenced by antibiotic resistance, as it seems that the pathogen is resistant to some but not all antibiotics. Additionally, C. perfringens is able to produce multiple toxins, however, they are differently structured but have the same target. This makes it easier for the pathogen to attack in various conditions, enabling the species to thrive in a greater range of ecosystems. Because of this, it is also able to affect animal hosts through their gut microbiota as they are influential on microbiota composition. Because of these characteristics, it enables the pathogen C. perfringens to cause more potential harm. This also helps learn more about food-borne illness as this is the one of the more common examples of the illness. Therefore, in public health, it is very important to understand how to prevent and build new treatment methods this common food-borne pathogen as a way to keep people safe.
References
- ↑ 1.0 1.1 Nyachuba, D. G. 2010. Foodborne illness: is it on the rise?: Nutrition Reviews©, Vol. 68, No. 5. Nutrition Reviews 68:257–269.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Uzal, F. A., J. C. Freedman, A. Shrestha, J. R. Theoret, J. Garcia, M. M. Awad, V. Adams, R. J. Moore, J. I. Rood, and B. A. McClane. 2014. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiology 9:361–377.
- ↑ 3.0 3.1 3.2 CDC, Prevent Illness From C. perfringens
- ↑ Rood, J. I., V. Adams, J. Lacey, D. Lyras, B. A. McClane, S. B. Melville, R. J. Moore, M. R. Popoff, M. R. Sarker, J. G. Songer, F. A. Uzal, and F. Van Immerseel. 2018. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 53:5–10.
- ↑ [https://www.sciencedirect.com/science/article/pii/S1075996415301001?via%3Dihub/ Ngamwongsatit, B., W. Tanomsridachchai, O. Suthienkul, S. Urairong, W. Navasakuljinda, and T. Janvilisri. 2016. Multidrug resistance in Clostridium perfringens isolated from diarrheal neonatal piglets in Thailand. Anaerobe 38:88–93. .
- ↑ 6.0 6.1 [https://dx.plos.org/10.1371/journal.pone.0205784/ Yang, W.-Y., Y. Lee, H. Lu, C.-H. Chou, and C. Wang. 2019. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 14:e0205784.
- ↑ Yang, Q., J. Liu, X. Wang, K. Robinson, M. A. Whitmore, S. N. Stewart, J. Zhao, and G. Zhang. 2021. Identification of an Intestinal Microbiota Signature Associated With the Severity of Necrotic Enteritis. Front. Microbiol. 12:703693.
- ↑ [https://journals.asm.org/doi/10.1128/AAC.31.2.312/ Stevens, D. L., B. M. Laine, and J. E. Mitten. 1987. Comparison of single and combination antimicrobial agents for prevention of experimental gas gangrene caused by Clostridium perfringens. Antimicrob Agents Chemother 31:312–316.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
