Commercial applications of Rhodopseudomonas palustris
Introduction
Rhodopseudomonas palustris is an Alphaproteobacteria under the genus Rhodopseudomonas. R. palustris is a non-sulfur purple bacterium that is gram-negative with a rod shape. Cells are motile and the organism reproduces by means of budding. R. palustris is found in both aerobic and anaerobic environments including a wide variety of marine and soil ecosystems; coastal marine sediment and waste water treatment facilities for example[1]. Typically non-sulfur purple bacteria are phototrophic, however R. palustris has the capability to switch between different forms of metabolism depending on environmental conditions: photoautotrophy, photoheterotrophy, chemoautotrophy, and chemoheterotrophy.
Rhodopseudomonas palustris is the focus of extensive research because it has a multitude of potential metabolic processes. Research focusing on bioremediation and removal of waste is interested in R. palustris for its potential to break down aromatic compounds and waste in polluted environments. Rhodopseudomonas also has the capability to produce hydrogen as a product of nitrogen fixation, which researchers are looking into as a form of alternate energy produced from biofuel. R. palustris has also displayed the capability to generate electricity further increasing the scope of research surrounding the microbe. Climate change induced by global warming and pollution of environments are some of the largest environmental problems facing the modern world. R. palustris is interesting in that it could hold the potential to solving not just one, but both of these serious environmental issues. The extensive research on this microbe presents a possibility for unique commercial applications of the microbe as a source of alternate energy and in waste management and removal.
Metabolism
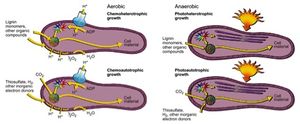
R. palustris has the capability to metabolize under aerobic and anaerobic conditions, meaning the organism can produce energy with or without oxygen present. Anaerobically, R. palustris can utilize photoheterotrophy and photoautotrophy, or photosynthesis[2]. The cell has the capability to fix carbon dioxide as biomass through photosynthesis, similar to green plants, but also R. palustris has the capability to obtain energy from light and other organic carbon forms via photoheterotrophy[3]. Under photoheterotrophic conditions, R. palustris utilizes organic compounds, most notably lignin monomers as a source of carbon[4]. When the cell is in the presence of conditions conducive to phototrophy, lamellar intracytoplasmic membranes hold bacteriochlorophylls and carotenoids, the photosynthetic pigments of the cell that make up the light harvesting complex, next to the cell membrane, which allows sunlight to be absorbed by the photosynthetic pigments[5]. The light energy absorbed is used to move electrons eventually resulting in the production of adenosine triphosphate (ATP). ATP is used as energy within the cell while the fixation of carbon dioxide or other organic compounds allows for the production of biomass and new cell material. Research has found that, R. palustris is able to respond to varying levels of light in its environment; in environments with lower light intensity the cell increases the amount of light harvesting complexes present to more effectively capture light energy[6]. Like photosynthetic plants, R. palustris possesses genes, which encode for RuBisCo, the enzyme responsible for the fixation of carbon dioxide in plants[7]. RuBisCo is used for the same fixation function in R. palustris photosynthesis, however unlike in plant photosynthesis oxygen is not a product of photosynthesis, as water is not split in the reaction and R. palustris utilizes thiosulfate, hydrogen and other inorganic electron donors[8].
Research has shown that R. palustris has genes that resemble genes found in oxygenic photosynthetic bacteria that regulate a form of circadian clock for the organism[9]. While photosynthesis generates large amounts of energy for the cell during light hours, the energy produced would be limited during dark hours. The presence of these circadian clock genes would suggest that there is circadian regulation of reactions, in the cell notably for the process of nitrogen fixation[10]. Rhodopseudomonas is an example of a microbe that performs anoxic photosynthesis in which oxygen is not generated and instead nitrogen gas is fixed. R. palustris possesses genes that encode nitrogenase enzymes, which allows the organisms to take in atmospheric nitrogen gas and fix the nitrogen into ammonia and other fixed nitrogen forms.
When oxygen is present in the environment, R. palustris can utilize forms of chemotrophy for metabolizing energy in the cell. These processes are regulated by the abundance of compounds present in the environment, as R. palustris has the flexibility to metabolize as a chemoautotroph or as a chemoheterotroph in the presence of oxygen[11]. As a chemoautotroph, R. palustris uses inorganic organic electron donors, like thiosulfate and hydrogen, to synthesis organic compounds using carbon dioxide as an oxidizer of the inorganic electron donor[12]. If carbon dioxide is not present in the system, or is present in small amounts, R. palustris can synthesize other organic compounds, namely lignin, in order for the cell to form its own organic compounds for use in the cell. Both of these metabolic forms in R. palustris utilize proton motive force, a hydrogen ion gradient in and around the cell, to transform adenosine diphosphate into adenosine triphosphate to provide the energy required within the cell. Furthermore, both chemotrophic metabolic processes result in the production of water, since oxygen serves as the final electron acceptor of the system. Research has shown however that R. palustris can utilize dimethyl sulfoxide, potassium nitrate, or sodium nitrite as terminal electron acceptors instead of oxygen[13].
Biodegredation
The function of most importance in studying R. palustris capabilities as a biodegradation tool is its ability to breakdown aromatic compounds. Aromatic structures, on a most basic level defined by having a planar ring, are commonly found in many, if not most, of the pollutants that can be harmful to environmental systems. Industrial wastes, sewage, plastics, and pesticides used in agriculture are all common pollutants and all contain aromatic structures of some form. One of the most common forms of aromatic pollutants is benzene, which is found in many commercial and waste products. The danger of benzene and many other aromatics is the ease of solubility, making it easy for these pollutants to enter water systems and cause harm[14] . These aromatic pollutants pose a serious threat to the health of many ecosystems and can also have adverse health consequences when they come into human contact.
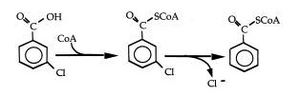
The ability of R. palustris to degrade aromatic compounds has been researched extensively. This research has lead to a fairly complete understanding of the mediated pathway used to breakdown 3-chlorobenzoate, a common aromatic form observed in many pollutants. R. palustris utilizes a reductive coenzyme to convert, via reduction and dehalogentation, 3-chlorobenzoate into acetyl CoA and carbon dioxide[15] . In chemotrophic metabolism, the cell utilizes acetyl CoA as an energy source, incorporating the newly formed acetyl CoA compound into the tricarboxylic acid cycle. This process is unique in R. palustris in that the aromatic compound, in the pathway of 3-chlorobenzoate degradation, is the substrate for complete dehalogenation[16] . Further research has shown that R. palustris has been able to reductively dehalogenated and assimilate many different forms of aromatic acids, phenolic, dihydroxylated, and methoxylated, as well as aromatic aldehydes, and hydroaromatic acids as R. palustris growth has been observed on these substrates with and without the presence of oxygen[17] . R. palustris has also demonstrated the ability to breakdown halocarboxylic acids; another common environmental pollutant found in aquatic and moist soil environments[18] .
R. palustris degradation of aromatics is unique not just because of the diversity of the aromatics it can breakdown, but R. palustris is also unique in the multitude of metabolic processes it can utilize to break down the aromatic compounds. In fact research has shown that even strains of R. palustris with similar substrate utilization patterns can have differences in degredation[19] . Furthermore, research indicates that R. palustris can utilize its different metabolic pathways, aerobic and anaerobic, to mediate the breakdown of a wide range of aromatic compounds[20] . The ability to breakdown these pollutant aromatic structures is important to understand commercially, as this capability of R. palustris could prove to be harnessed and utilized in order to remove a wide range of pollutants from many different environments. The diversity of aromatics that can be degraded allows for wide coverage of possible pollutants R. palustris can break down. The ability to utilize different metabolic pathways to degrade the aromatics allows the microbe to remove pollutants in a wide range of environments as well. Research has already been conducted indicating the success of R. palustris removing odorous pollutants from wastewater lagoons[21] and eutrophic ponds[22] .
Electricity Generation
Include some current research, with at least one figure showing data.
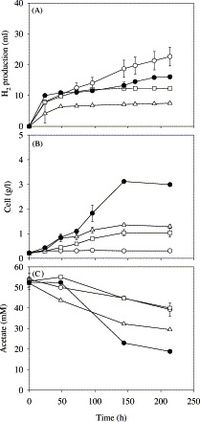
Hydrogen Production
Conclusion
References
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Mehrabi,S. et al." Identification and characterization of Rhodopseudomonas spp., a purple, non-sulfur bacterium from microbial mats"."Biomolecular Engineering". 2001.Volume 18,p.49-56.
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Scheuring, S. “The Photosynthetic Apparatus of Rhodopseudomonas palustris: Structures and Organization”. Journal of Molecular Biology. 2006.Volume 358, Issue 1, p.83-96.
- ↑ Scheuring, S. “The Photosynthetic Apparatus of Rhodopseudomonas palustris: Structures and Organization”. Journal of Molecular Biology. 2006.Volume 358, Issue 1, p.83-96.
- ↑ Scheuring, S. “The Photosynthetic Apparatus of Rhodopseudomonas palustris: Structures and Organization”. Journal of Molecular Biology. 2006.Volume 358, Issue 1, p.83-96.
- ↑ Scheuring, S. “The Photosynthetic Apparatus of Rhodopseudomonas palustris: Structures and Organization”. Journal of Molecular Biology. 2006.Volume 358, Issue 1, p.83-96.
- ↑ [ http://aem.asm.org/content/67/3/1396.full Egland, P. et al. “Reductive Coenzyme A-Mediated Pathway for 3-Chlorobenzoate Degradation in the Phototrophic Bacterium Rhodopseudomonas palustris”. Applied and Environmental Microbiology. 2001. Volume 67, Issue 3, p. 1396-1399.]
- ↑ Larimer F. et al. “Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris”. Nature Biotechnology. 2004. Volume 22, p.55-61
- ↑ Lang, F., Oesterhelt, D. "Microaerophilic growth and induction of the photosynthetic reaction center in Rhodopseudomonas viridis." Journal of Bacteriology, 1989. Volume 171, Issue 5. American Society for Microbiology. (2327-2834).
- ↑ [ http://aem.asm.org/content/67/3/1396.full Egland, P. et al. “Reductive Coenzyme A-Mediated Pathway for 3-Chlorobenzoate Degradation in the Phototrophic Bacterium Rhodopseudomonas palustris”. Applied and Environmental Microbiology. 2001. Volume 67, Issue 3, p. 1396-1399.]
- ↑ [ http://aem.asm.org/content/67/3/1396.full Egland, P. et al. “Reductive Coenzyme A-Mediated Pathway for 3-Chlorobenzoate Degradation in the Phototrophic Bacterium Rhodopseudomonas palustris”. Applied and Environmental Microbiology. 2001. Volume 67, Issue 3, p. 1396-1399.]
- ↑ [ http://aem.asm.org/content/67/3/1396.full Egland, P. et al. “Reductive Coenzyme A-Mediated Pathway for 3-Chlorobenzoate Degradation in the Phototrophic Bacterium Rhodopseudomonas palustris”. Applied and Environmental Microbiology. 2001. Volume 67, Issue 3, p. 1396-1399.]
- ↑ Harwood, C., Gibson, J. ”Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris.” Applied and Environmental Microbiology. 1988. Volume 54, Issue 3, p. 712-717.
- ↑ McGrath, J., Harfoot, C. “Reductive dehalogenation of halocarboxylic acids by the phototrophic genera Rhodospirillum and Rhodopseudomonas”. Applied and Environmental Microbiology. 1997. Volume 63, Issue 8, p. 3333-3335.
- ↑ Harwood, C., Gibson, J. ”Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris.” Applied and Environmental Microbiology. 1988. Volume 54, Issue 3, p. 712-717.
- ↑ Harwood, C., Gibson, J. ”Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris.” Applied and Environmental Microbiology. 1988. Volume 54, Issue 3, p. 712-717.
- ↑ Sharma, N. et al. “Skatole remediation potential of Rhodopseudomonas palustris WKU-KDNS3 isolated from an animal waste lagoon”. Lett Appl Microbiol. 2015. Volume 60, Issue 3, p. 298-306.
- ↑ Myung, K. et al. “Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds”. Biotechnology Letters. 2004. Volume 26, Issue 10, p. 819-822.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
