Copper Mining Using Acidothiobacillus: Difference between revisions
GipsonAndrew (talk | contribs) |
No edit summary |
||
| (54 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
[[Image:BioleachingPlantKCCLUganda.jpg|thumb|400px|right|Figure 1. A copper bioleaching plant in Uganda. (http://promine.gtk.fi/about/WorkPackage4.html)]] | [[Image:BioleachingPlantKCCLUganda.jpg|thumb|400px|right|Figure 1. A copper bioleaching plant in Uganda. (http://promine.gtk.fi/about/WorkPackage4.html)]] | ||
=Introduction= | |||
By Andrew Gipson<br> | By Andrew Gipson<br> | ||
<br>Copper has been one of mankind's most important metal resources since the beginning of civilization, and to this day it holds and important role in the functioning of modern society. It's main uses include piping, coinage, electronics, and even antibiotics (Wikipedia: | <br>Copper has been one of mankind's most important metal resources since the beginning of civilization, and to this day it holds and important role in the functioning of modern society. It's main uses include piping, coinage, electronics, and even antibiotics (Wikipedia: Copper [http://en.wikipedia.org/wiki/Copper]). In 2009, global copper consumption topped 21 billion tons (Copper Development Association), and that kind of demand has led to the development of sophisticated mining techniques.<br> | ||
Copper is rarely found in its native form; most often it exists as ores made up of various copper sulfides and oxides that have little practical value of their own (Australian Atlas of Minerals, Resources, and Processing Centres). As such, methods for mining copper involve long and complex chemical processes to produce metal from the ores. This involves using harsh chemical reagents to pull the ore from the rock, and subsequent treatment to further isolate the metal and extract pure copper | <br>Copper is rarely found in its native form; most often it exists as ores made up of various copper sulfides and oxides that have little practical value of their own (Australian Atlas of Minerals, Resources, and Processing Centres). As such, methods for mining copper involve long and complex chemical processes to produce metal from the ores. This involves using harsh chemical reagents to pull the ore from the rock, and subsequent treatment to further isolate the metal and extract pure copper. These processes are energetically expensive, and they create a significant amount of pollutant chemicals such as sulfur dioxide and cyanide (Copper Mining Info [http://www.mine-engineer.com/mining/copperm.htm]).<br> | ||
A viable alternative to these harmful procedures is biomining, or the use of bacterial metabolic processes to assist in the solubilization of metal ores. <i>Acidothiobacillus ferrooxidans</i> is a lithoautotroph famous for using ferrous iron (Fe<sup>2+</sup>) as an electron donor along with elemental sulfur. They grow in subterranean mineral deposits, and their activity has been linked to the processing of mineral ores (Rawlings 2002). | <br>A viable alternative to these harmful procedures is biomining, or the use of bacterial metabolic processes to assist in the solubilization of metal ores. <i>Acidothiobacillus ferrooxidans</i> is a lithoautotroph famous for using ferrous iron (Fe<sup>2+</sup>) as an electron donor along with elemental sulfur. They grow in subterranean mineral deposits and even sewer pipes (Slonczewski and Foster 2011), and their activity has been linked to the processing of mineral ores (Rawlings 2002). | ||
=Copper Bioleaching Techniques= | |||
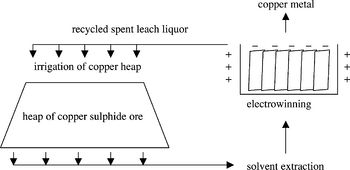
[[Image:Rawlings 2002 Heap leaching of copper-containing ore.jpeg|thumb|350px|right|Figure 2. Outline of the heap leaching process. After irrigating the heap with a lixiviant, the copper-containing leach liquor is collected and processed by solvent extraction and electrowinning to yield copper metal. (Rawlings 2002)]] | |||
Modern biomining processes are large-scale operations that involve the mining of ores and processing for several months (Fig. 1). Either through strip-mining or blasting, raw ore is obtained for extraction of copper. | |||
===Heap Irrigation=== | |||
The ores obtained from mining are crushed and piled up to 10 meters high atop plastic irrigation pads, whereupon water seeded with acid, called the lixiviant, is irrigated through the heaps and collected (Fig. 2). After performing the next steps to extract the copper dissolved in the leach liquor, it is recycled and used to irrigate the heap again (Rawlings 2002).<br> | |||
===Copper Extraction from Leach Liquor=== | |||
The isolation of copper metal from leach liquor is a two-step process (Wikipedia: Copper Extraction Techniques [http://en.wikipedia.org/wiki/Copper_extraction_techniques]). The leach liquor obtained after heap irrigation has a very low concentration of copper dissolved into it, around 0.5 - 2.0 g/L. Through solvent extraction, the copper is transferred from the dilute liquor to a more concentrated solution. Afterward, the dissolved copper ions are transformed to solid copper metal through a process called electrowinning that uses an electric current to reduce the dissolved metal and deposit it onto a cathode, at which point the solid elemental copper metal can be employed for its many uses (Siddiqui <i>et al.</i> 2009). | |||
=Mechanism of Bacterial Leaching= | |||
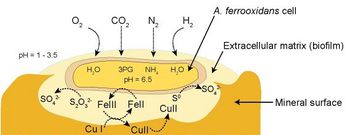
[[Image:At_ferrooxidans_EPS.jpg|thumb|350px|right|Figure | [[Image:At_ferrooxidans_EPS.jpg|thumb|350px|right|Figure 3. <i>At. ferrooxidans</i> binds to a metal sulfide substrate by way of an extracellular polymer matrix. The redox cycling conducted in the matrix helps to sustain the bacterium. (Valdés <i>et al.</i> 2008)]] | ||
There has been much debate over how <i>At. ferrooxidans</i> contributes to the conversion of metal ores into soluble compounds. Although it has been proposed that the bacterium produces enzymes which directly mediate the reaction, studies have shown that iron is the only metal ion used to produce energy (Mignone and Donati 2004). Copper is not a metabolite of <i>At. ferrooxidans</i>, so the bacteria is not directly responsible for the solubilization of copper. Rather, it occurs through an abiotic reaction between the copper ores and the ferric ions and protons produced by the metabolic activities of the bacteria. These reactions regenerate the reduced iron and sulfur compounds that the bacteria reuptake to start the cycle anew.<br> | There has been much debate over how <i>At. ferrooxidans</i> contributes to the conversion of metal ores into soluble compounds. Although it has been proposed that the bacterium produces enzymes which directly mediate the reaction, studies have shown that iron is the only metal ion used to produce energy (Mignone and Donati 2004). Copper is not a metabolite of <i>At. ferrooxidans</i>, so the bacteria is not directly responsible for the solubilization of copper. Rather, it occurs through an abiotic reaction between the copper ores and the ferric ions and protons produced by the metabolic activities of the bacteria. These reactions regenerate the reduced iron and sulfur compounds that the bacteria reuptake to start the cycle anew.<br> | ||
===Bacterial Metabolic Reactions=== | ===Bacterial Metabolic Reactions=== | ||
| Line 23: | Line 31: | ||
Copper oxides like azurite are somewhat less susceptible to attack by ferric iron; however, they are highly soluble in the low-pH solution produced by the bacterial production of sulfuric acid (Iasillo 1999):<br> | Copper oxides like azurite are somewhat less susceptible to attack by ferric iron; however, they are highly soluble in the low-pH solution produced by the bacterial production of sulfuric acid (Iasillo 1999):<br> | ||
<center>Cu<sub>3</sub>(OH)<sub>2</sub>(CO<sub>3</sub>)<sub>4</sub> (azurite) + 3H<sub>2</sub>O --> 3CuSO<sub>4</sub> + CO<sub>2</sub> + 4H<sub>2</sub>O</center><br> | <center>Cu<sub>3</sub>(OH)<sub>2</sub>(CO<sub>3</sub>)<sub>4</sub> (azurite) + 3H<sub>2</sub>O --> 3CuSO<sub>4</sub> + CO<sub>2</sub> + 4H<sub>2</sub>O</center><br> | ||
This cycling of iron and sulfur maintains an energetically favorable situation for the bacteria; any ferric iron or sulfuric acid produced tends to undergo reduction with mineral ores to be subsequently re-oxidized by <i>At. ferrooxidans</i>. This cycling does not occur in a free-floating environment, however; the bacteria produce an extracellular biofilm made of polysaccharides and lipids which binds them to the mineral substrate and retains the iron and sulfur compounds as they go through the redox cycle (Fig. | This cycling of iron and sulfur maintains an energetically favorable situation for the bacteria; any ferric iron or sulfuric acid produced tends to undergo reduction with mineral ores to be subsequently re-oxidized by <i>At. ferrooxidans</i>. This cycling does not occur in a free-floating environment, however; the bacteria produce an extracellular biofilm made of polysaccharides and lipids which binds them to the mineral substrate and retains the iron and sulfur compounds as they go through the redox cycle (Fig. 3). The biofilm also helps to retain the bacterial cultures in heaps during irrigation (Sand and Gehrke 2006). | ||
==Improvements to the Biomining Process== | |||
===Improving the Organism=== | |||
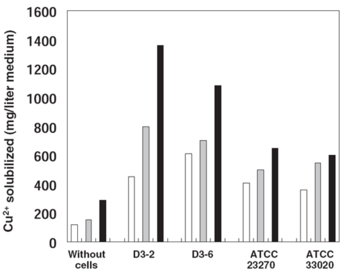
[[Image:Sugio_et_al_2008_Solubilization_of_Cu2+_from_a_Chalcopyrite_Concentrate.png|thumb|350px|right|Figure 4. Concentrations of copper solubilized from chalcopyrite in low-pH cultures of different <i>At. ferrooxidans</i> strains. White bar = 7 d after inoculation, gray bar = 14 d, and black bar = 30 d. (Sugio <i>et al.</i> 2008)]]A study done on the copper solubilizing activity of <i>At. ferrooxidans</i> strains isolated from a low-grade copper mine in Chile revealed that when grown in a chalcopyrite-containing medium, a particular strain called D3-2 had a greatly enhanced solubilizing activity in relation to other strains (Fig. 4). The reason for this increased activity is connected to the D3-2 strain's resistance to sulfite (SO<sub>3</sub><sup>2-</sup>), a toxic intermediate in sulfur oxidation. Sulfite hinders the activity of iron oxidase, an important enzyme in the bacterium's iron oxidation pathway which provides ferric ions for the solubilization of sulfides (Sugio <i>et al.</i> 1994). Most organisms including <i>At. ferrooxidans</i> possess a sulfite oxidase enzyme that converts sulfite to sulfate under low sulfite conditions (Feng ''et al.'' 2007), but this activity tends to be inhibited at higher concentrations. However, the D3-2 strain has a sulfite reductase that is more resistant to sulfite and thus can oxidize it faster, resulting in a lowered sulfite concentration that doesn't inhibit the iron oxidation pathway (Sugio <i>et al.</i> 2008).<br> | |||
One cause for concern regarding the efficiency of the process in regards to the organism is the makeup of the microbial community in a heap reactor. Studies on the composition of the bacterial community have showed that under lower-nutrient conditions and in the presence of certain toxic metals such as arsenic ''At. ferrooxidans'' is not the majority member, but rather non-iron oxidizing bacteria such as ''Thiobacillus caldus'' (Rawlings ''et al.'' 1999). This is problematic because the metabolic activities of these other species do not produce the ferric ions responsible for solubilizing copper sulfates. Improvement of the nutrient conditions in the heap reactor can increase the ''At. ferrooxidans'' population, which can be achieved through the addition of phosphate (Varela ''et al.'' 1998). | |||
== | ===Improving the Process=== | ||
A more inexpensive and immediately practical way than genetic modification to improve biomining is through optimization of industrial techniques. <i>In situ</i> biomining does away with the ore mining process altogether; acidified leach liquor is pumped directly into the ground and the copper is solubilized utilizing preexisting bacteria in the ore deposits. The copper-rich liquor is extracted from wells dug beneath the deposits (Rawlings 2002). By eliminating the physical act of ore mining, a significant portion of the costs is cut from the process. | |||
"Copper." ''Wikipedia''.[http://www.copper.org/education/c-facts/homepage.html]<br> | =Conclusion= | ||
Bioming has proved to be a cheaper, more efficient, and more environmentally-friendly alternative than non-biologically mediated techniques for copper mining, and the chemolithotrophic bacteria ''At. ferrooxidans'' has proven to be an essential part of this process. Much research is still underway to develop improvements, and future applications have included using biologically-engineered bacteria to mine extraterrestrial bodies such as the moon and large meteors (Ragozzine 2004). Whatever directions biomining will take in the future, it will doubtlessly prove to be immensely beneficial in a future with dwindling resources. | |||
"Copper Extraction Techniques." ''Wikipedia''. [http:// | =References= | ||
Copper Mining Info [http://www. | [http://www.australianminesatlas.gov.au/education/fact_sheets/copper.jsp Australian Atlas of Minerals, Resources, and Processing Centres.]<br> | ||
[http://www.biobasics.gc.ca/english/View.asp?x=797 Biomining. ''Biobasics''. Retrieved April 30, 2011.]<br> | |||
[http://en.wikipedia.org/wiki/Copper "Copper." ''Wikipedia''.]<br> | |||
[http://www.copper.org/education/c-facts/homepage.html Copper Development Association]<br> | |||
[http://en.wikipedia.org/wiki/Copper_extraction_techniques "Copper Extraction Techniques." ''Wikipedia''.]<br> | |||
[http://www.mine-engineer.com/mining/copperm.htm Copper Mining Info ]<br> | |||
[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1993547/?tool=pmcentrez Feng C, Tollin G, Enemark JH (2007) Sulfite oxidizing enzymes. ''Biochim Biophys Acta.'' <b>1774</b>(5): 527–539.]<br> | |||
[http://books.google.com/books?id=2uvHg43YTiUC&pg=PA125&lpg=PA125&dq=acid+solubility+of+copper+minerals&source=bl&ots=ZfCHMw_8qX&sig=ae3F58Qvvo1q8YKFoXtj1Polknw&hl=en&ei=Ct-1TaC-O4P00gHr2KCrCQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBUQ6AEwAA#v=onepage&q&f=true Iasillo E (1999) Metallurgical test program for copper leaching projects. In ''Copper Leaching, Extraction, and Electrowinning Techniques'', ed. Jergensen GV. 124-128. Littleton: SME.]<br> | [http://books.google.com/books?id=2uvHg43YTiUC&pg=PA125&lpg=PA125&dq=acid+solubility+of+copper+minerals&source=bl&ots=ZfCHMw_8qX&sig=ae3F58Qvvo1q8YKFoXtj1Polknw&hl=en&ei=Ct-1TaC-O4P00gHr2KCrCQ&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBUQ6AEwAA#v=onepage&q&f=true Iasillo E (1999) Metallurgical test program for copper leaching projects. In ''Copper Leaching, Extraction, and Electrowinning Techniques'', ed. Jergensen GV. 124-128. Littleton: SME.]<br> | ||
Leduc LG and Ferroni GD (1994) The chemolithotrophic bacterium ''Thiobacillus ferrooxidans''. ''FEMS Microbiology Reviews'' '''14''':103-120.<br> | Leduc LG and Ferroni GD (1994) The chemolithotrophic bacterium ''Thiobacillus ferrooxidans''. ''FEMS Microbiology Reviews'' '''14''':103-120.<br> | ||
[http://journals.ohiolink.edu/ejc/pdf.cgi/Mignone_C.F.pdf?issn=1369703x&issue=v18i0003&article=211_arfgamoib Mignone CF and Donati ER (2004)ATP requirements for growth and maintenance of iron-oxidizing bacteria. ''Biochemical Engineering Journal'' '''18''': 211–216.]<br> | [http://journals.ohiolink.edu/ejc/pdf.cgi/Mignone_C.F.pdf?issn=1369703x&issue=v18i0003&article=211_arfgamoib Mignone CF and Donati ER (2004) ATP requirements for growth and maintenance of iron-oxidizing bacteria. ''Biochemical Engineering Journal'' '''18''': 211–216.]<br> | ||
Rawlings DE (2002) Heavy metal mining using microbes. ''Annu. Rev. Microbiol.'' '''56''': 65-91.<br> | Rawlings DE, Coram NJ, Gardner MN, Deane SM (1999) ''Thiobacillus caldus'' and ''Leptospirillum ferrooxidans'' are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates. In ''Biohydrometallurgy and the Environment Towards the 21st Century. Part A'', ed. Amils R and Ballester A. 777-786. Amsterdam: Elsevier<br> | ||
[http://www.annualreviews.org/doi/full/10.1146/annurev.micro.56.012302.161052?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed& Rawlings DE (2002) Heavy metal mining using microbes. ''Annu. Rev. Microbiol.'' '''56''': 65-91.]<br> | |||
[http://www.niac.usra.edu/files/library/meetings/fellows/mar04/Ragozzine_Darin.pdf Rizzone D (2004) Biomining for ''In-Situ'' Resource Utilization. Accessed May 2011.]<br> | |||
Sand W and Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via | Sand W and Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via | ||
interfacial processes involving iron(III) ions and acidophilic bacteria. ''Research in Microbiology'' '''157''': 49-56. | interfacial processes involving iron(III) ions and acidophilic bacteria. ''Research in Microbiology'' '''157''': 49-56.<br> | ||
Valdés J, Pedroso I, Quatrini R, Dodson RJ,Tettelin H, Blake R, Eisen JA, Holmes DS (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. ''BMC Genomics'' '''9''': 597.<br> | Siddiqui MH, Kumar A, Kesari KK, Arif JM (2009) Biomining: A useful approach toward metal extraction. ''American-Eurasian Journal of Agronomy'' '''2'''(2): 84-88<br> | ||
Slonczewski JL, Foster JW (2011) ''Microbiology: An Evolving Science''. 2<sup>nd</sup> Ed. New York: W.W. Norton & Company. 1097 p.<br> | |||
Sugio T, Iwahori K, Uemura S, Makino I, and Tano T (1994) Inhibition of iron-oxidizing activity by bisulfite ion in | |||
Thiobacillus ferrooxidans. ''Biosci. Biotech. Biochem.'' '''58''': 2109–2110.<br> | |||
Sugio T, Wakabayashi M, Kanao T, Takeuchi F (2008) Isolation and Characterization of Acidithiobacillus ferrooxidans Strain D3-2 Active in Copper Bioleaching from a Copper Mine in Chile. ''Biosci. Biotechnol. Biochem.'' '''72'''(4): 998–1004.<br> | |||
Valdés J, Pedroso I, Quatrini R, Dodson RJ, Tettelin H, Blake R, Eisen JA, Holmes DS (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. ''BMC Genomics'' '''9''': 597.<br> | |||
Varela P, Levican G, Rivera F, Jerez CA (1998) An immunological strategy to monitor in situ the phosphate starvation state in ''Thiobacillus ferrooxidans''. ''Applied and Environmental Microbio.'' '''64'''(12): 4990-4993. | |||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 14:30, 23 July 2011

Introduction
By Andrew Gipson
Copper has been one of mankind's most important metal resources since the beginning of civilization, and to this day it holds and important role in the functioning of modern society. It's main uses include piping, coinage, electronics, and even antibiotics (Wikipedia: Copper [1]). In 2009, global copper consumption topped 21 billion tons (Copper Development Association), and that kind of demand has led to the development of sophisticated mining techniques.
Copper is rarely found in its native form; most often it exists as ores made up of various copper sulfides and oxides that have little practical value of their own (Australian Atlas of Minerals, Resources, and Processing Centres). As such, methods for mining copper involve long and complex chemical processes to produce metal from the ores. This involves using harsh chemical reagents to pull the ore from the rock, and subsequent treatment to further isolate the metal and extract pure copper. These processes are energetically expensive, and they create a significant amount of pollutant chemicals such as sulfur dioxide and cyanide (Copper Mining Info [2]).
A viable alternative to these harmful procedures is biomining, or the use of bacterial metabolic processes to assist in the solubilization of metal ores. Acidothiobacillus ferrooxidans is a lithoautotroph famous for using ferrous iron (Fe2+) as an electron donor along with elemental sulfur. They grow in subterranean mineral deposits and even sewer pipes (Slonczewski and Foster 2011), and their activity has been linked to the processing of mineral ores (Rawlings 2002).
Copper Bioleaching Techniques
Modern biomining processes are large-scale operations that involve the mining of ores and processing for several months (Fig. 1). Either through strip-mining or blasting, raw ore is obtained for extraction of copper.
Heap Irrigation
The ores obtained from mining are crushed and piled up to 10 meters high atop plastic irrigation pads, whereupon water seeded with acid, called the lixiviant, is irrigated through the heaps and collected (Fig. 2). After performing the next steps to extract the copper dissolved in the leach liquor, it is recycled and used to irrigate the heap again (Rawlings 2002).
Copper Extraction from Leach Liquor
The isolation of copper metal from leach liquor is a two-step process (Wikipedia: Copper Extraction Techniques [3]). The leach liquor obtained after heap irrigation has a very low concentration of copper dissolved into it, around 0.5 - 2.0 g/L. Through solvent extraction, the copper is transferred from the dilute liquor to a more concentrated solution. Afterward, the dissolved copper ions are transformed to solid copper metal through a process called electrowinning that uses an electric current to reduce the dissolved metal and deposit it onto a cathode, at which point the solid elemental copper metal can be employed for its many uses (Siddiqui et al. 2009).
Mechanism of Bacterial Leaching
There has been much debate over how At. ferrooxidans contributes to the conversion of metal ores into soluble compounds. Although it has been proposed that the bacterium produces enzymes which directly mediate the reaction, studies have shown that iron is the only metal ion used to produce energy (Mignone and Donati 2004). Copper is not a metabolite of At. ferrooxidans, so the bacteria is not directly responsible for the solubilization of copper. Rather, it occurs through an abiotic reaction between the copper ores and the ferric ions and protons produced by the metabolic activities of the bacteria. These reactions regenerate the reduced iron and sulfur compounds that the bacteria reuptake to start the cycle anew.
Bacterial Metabolic Reactions
At. ferrooxidans has a diverse set of metabolic processes that allow it to be lithoautotrophic, but the most economically important activity this bacterium performs is the oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+):
...and the oxidation of elemental sulfur (So) to sulfuric acid (H2SO4):
These respiratory pathways produce the reactive species that initiate mineral solubilization (Leduc and Ferroni 1994).
Abiotic Solubilization and Regeneration of Bacterial Substrates
Copper sulfide ores are solubilized in a reaction where ferric iron complexed with sulfate attacks the copper compounds and becomes reduced to ferrous iron while generating copper sulfates and elemental sulfur (Hutchins et al. 1986):
Copper oxides like azurite are somewhat less susceptible to attack by ferric iron; however, they are highly soluble in the low-pH solution produced by the bacterial production of sulfuric acid (Iasillo 1999):
This cycling of iron and sulfur maintains an energetically favorable situation for the bacteria; any ferric iron or sulfuric acid produced tends to undergo reduction with mineral ores to be subsequently re-oxidized by At. ferrooxidans. This cycling does not occur in a free-floating environment, however; the bacteria produce an extracellular biofilm made of polysaccharides and lipids which binds them to the mineral substrate and retains the iron and sulfur compounds as they go through the redox cycle (Fig. 3). The biofilm also helps to retain the bacterial cultures in heaps during irrigation (Sand and Gehrke 2006).
Improvements to the Biomining Process
Improving the Organism
A study done on the copper solubilizing activity of At. ferrooxidans strains isolated from a low-grade copper mine in Chile revealed that when grown in a chalcopyrite-containing medium, a particular strain called D3-2 had a greatly enhanced solubilizing activity in relation to other strains (Fig. 4). The reason for this increased activity is connected to the D3-2 strain's resistance to sulfite (SO32-), a toxic intermediate in sulfur oxidation. Sulfite hinders the activity of iron oxidase, an important enzyme in the bacterium's iron oxidation pathway which provides ferric ions for the solubilization of sulfides (Sugio et al. 1994). Most organisms including At. ferrooxidans possess a sulfite oxidase enzyme that converts sulfite to sulfate under low sulfite conditions (Feng et al. 2007), but this activity tends to be inhibited at higher concentrations. However, the D3-2 strain has a sulfite reductase that is more resistant to sulfite and thus can oxidize it faster, resulting in a lowered sulfite concentration that doesn't inhibit the iron oxidation pathway (Sugio et al. 2008).
One cause for concern regarding the efficiency of the process in regards to the organism is the makeup of the microbial community in a heap reactor. Studies on the composition of the bacterial community have showed that under lower-nutrient conditions and in the presence of certain toxic metals such as arsenic At. ferrooxidans is not the majority member, but rather non-iron oxidizing bacteria such as Thiobacillus caldus (Rawlings et al. 1999). This is problematic because the metabolic activities of these other species do not produce the ferric ions responsible for solubilizing copper sulfates. Improvement of the nutrient conditions in the heap reactor can increase the At. ferrooxidans population, which can be achieved through the addition of phosphate (Varela et al. 1998).
Improving the Process
A more inexpensive and immediately practical way than genetic modification to improve biomining is through optimization of industrial techniques. In situ biomining does away with the ore mining process altogether; acidified leach liquor is pumped directly into the ground and the copper is solubilized utilizing preexisting bacteria in the ore deposits. The copper-rich liquor is extracted from wells dug beneath the deposits (Rawlings 2002). By eliminating the physical act of ore mining, a significant portion of the costs is cut from the process.
Conclusion
Bioming has proved to be a cheaper, more efficient, and more environmentally-friendly alternative than non-biologically mediated techniques for copper mining, and the chemolithotrophic bacteria At. ferrooxidans has proven to be an essential part of this process. Much research is still underway to develop improvements, and future applications have included using biologically-engineered bacteria to mine extraterrestrial bodies such as the moon and large meteors (Ragozzine 2004). Whatever directions biomining will take in the future, it will doubtlessly prove to be immensely beneficial in a future with dwindling resources.
References
Australian Atlas of Minerals, Resources, and Processing Centres.
Biomining. Biobasics. Retrieved April 30, 2011.
"Copper." Wikipedia.
Copper Development Association
"Copper Extraction Techniques." Wikipedia.
Copper Mining Info
Feng C, Tollin G, Enemark JH (2007) Sulfite oxidizing enzymes. Biochim Biophys Acta. 1774(5): 527–539.
Iasillo E (1999) Metallurgical test program for copper leaching projects. In Copper Leaching, Extraction, and Electrowinning Techniques, ed. Jergensen GV. 124-128. Littleton: SME.
Leduc LG and Ferroni GD (1994) The chemolithotrophic bacterium Thiobacillus ferrooxidans. FEMS Microbiology Reviews 14:103-120.
Mignone CF and Donati ER (2004) ATP requirements for growth and maintenance of iron-oxidizing bacteria. Biochemical Engineering Journal 18: 211–216.
Rawlings DE, Coram NJ, Gardner MN, Deane SM (1999) Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates. In Biohydrometallurgy and the Environment Towards the 21st Century. Part A, ed. Amils R and Ballester A. 777-786. Amsterdam: Elsevier
Rawlings DE (2002) Heavy metal mining using microbes. Annu. Rev. Microbiol. 56: 65-91.
Rizzone D (2004) Biomining for In-Situ Resource Utilization. Accessed May 2011.
Sand W and Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via
interfacial processes involving iron(III) ions and acidophilic bacteria. Research in Microbiology 157: 49-56.
Siddiqui MH, Kumar A, Kesari KK, Arif JM (2009) Biomining: A useful approach toward metal extraction. American-Eurasian Journal of Agronomy 2(2): 84-88
Slonczewski JL, Foster JW (2011) Microbiology: An Evolving Science. 2nd Ed. New York: W.W. Norton & Company. 1097 p.
Sugio T, Iwahori K, Uemura S, Makino I, and Tano T (1994) Inhibition of iron-oxidizing activity by bisulfite ion in
Thiobacillus ferrooxidans. Biosci. Biotech. Biochem. 58: 2109–2110.
Sugio T, Wakabayashi M, Kanao T, Takeuchi F (2008) Isolation and Characterization of Acidithiobacillus ferrooxidans Strain D3-2 Active in Copper Bioleaching from a Copper Mine in Chile. Biosci. Biotechnol. Biochem. 72(4): 998–1004.
Valdés J, Pedroso I, Quatrini R, Dodson RJ, Tettelin H, Blake R, Eisen JA, Holmes DS (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9: 597.
Varela P, Levican G, Rivera F, Jerez CA (1998) An immunological strategy to monitor in situ the phosphate starvation state in Thiobacillus ferrooxidans. Applied and Environmental Microbio. 64(12): 4990-4993.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.