Demodex folliculorum: Difference between revisions
No edit summary |
No edit summary |
||
| Line 26: | Line 26: | ||
==Section 1 Genetics== | ==Section 1 Genetics== | ||
[[Image:Follic.genome.jpg|thumb| | [[Image:Follic.genome.jpg|thumb|250px|right|Mitochondrial Genome of D. folliculorum and D. brevis. Protein-coding genes are green, rRNA genes are blue, tRNA genes are red. https://www.sciencedirect.com/science/article/pii/S0738081X14000467.]] | ||
D. folliculorum belong to the Animalia kingdom, the Arthropoda phylum, the Chelicerata subphylum, the Arachnida class, the Acariformes superorder, the Trombidiformes order, the Eleutherengonides supercohort, and the Demodicae family of mites. Estimates of the divergence of D. folliculorum and D. brevis range from 87-173 million of years ago. The mitochondrial genome of D. folliculorum is 14,150 base pairs long and on average 71% made up of AT-pairs. The genome includes thirteen protein-encoding genes, two rRNA genes, and twenty-two tRNA genes. Its Genbank accession number is KM114226.<ref>M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.</ref>. <br><br> | D. folliculorum belong to the Animalia kingdom, the Arthropoda phylum, the Chelicerata subphylum, the Arachnida class, the Acariformes superorder, the Trombidiformes order, the Eleutherengonides supercohort, and the Demodicae family of mites. Estimates of the divergence of D. folliculorum and D. brevis range from 87-173 million of years ago. The mitochondrial genome of D. folliculorum is 14,150 base pairs long and on average 71% made up of AT-pairs. The genome includes thirteen protein-encoding genes, two rRNA genes, and twenty-two tRNA genes. Its Genbank accession number is KM114226.<ref>M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.</ref>. <br><br> | ||
The D. folliculorum genome is nearly the same size as that of D. brevis (14,240 bp) and within the average range of other Acariformes. A likely explanation is that mites in this superorder face similar selection pressures that favor a compact genome for easier replication. The high amount of AT-pairs also improves metabolic efficiency as it requires less H-bonds to be broken in the formation of a replication fork. D. folliculorum shares the same number of protein-encoding, rRNA, and tRNA genes as all other Acariformes; however, their unique arrangement is only shared by D. brevis. D. folliculorum possesses several other recognizable traits of Acariformes, such as the readiness of movement of tRNA genes and the truncated form of these genes. It is believed that D. brevis and D. folliculorum inherited the same tRNA structures from a common ancestor as the average sizes of their tRNA genes (53.5 bp in D. brevis and 53.3 in D. folliculorum) are very close. Truncated tRNA genes found in both species also share their lack of T-arms and D-arms. Due to the divergence of D. brevis and D. folliculorum occurring many millions of years ago, scientists believe that selection pressure is maintaining these truncated genes. It possible they perform a novel function or can be repaired in some way.<ref>M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.</ref>. <br><br> | The D. folliculorum genome is nearly the same size as that of D. brevis (14,240 bp) and within the average range of other Acariformes. A likely explanation is that mites in this superorder face similar selection pressures that favor a compact genome for easier replication. The high amount of AT-pairs also improves metabolic efficiency as it requires less H-bonds to be broken in the formation of a replication fork. D. folliculorum shares the same number of protein-encoding, rRNA, and tRNA genes as all other Acariformes; however, their unique arrangement is only shared by D. brevis. D. folliculorum possesses several other recognizable traits of Acariformes, such as the readiness of movement of tRNA genes and the truncated form of these genes. It is believed that D. brevis and D. folliculorum inherited the same tRNA structures from a common ancestor as the average sizes of their tRNA genes (53.5 bp in D. brevis and 53.3 in D. folliculorum) are very close. Truncated tRNA genes found in both species also share their lack of T-arms and D-arms. Due to the divergence of D. brevis and D. folliculorum occurring many millions of years ago, scientists believe that selection pressure is maintaining these truncated genes. It possible they perform a novel function or can be repaired in some way.<ref>M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.</ref>. <br><br> | ||
Revision as of 03:19, 7 December 2019
Introduction
Demodex folliculorum are ecto-parasitic mites that live in human hair follicles. Commonly referred to as “eyelash mites”, D. folliculorum were discovered in 1842 by German scientist Gustav Simon who named the species Demodex, meaning lard-boring worm.[1]. Although the Demodex genus includes 65 different species of mites, D. folliculorum and D. Brevis are the only species in the Demodex genus that are found on humans. While D. brevis inhabit the sebaceous glands of hair follicles, D. folliculorum inhabit hair follicles themselves, particularly of eyelashes and eyebrows. They typically transfer hosts through close physical contact and increase in colonization as humans grow older. A skin surface biopsy (SSB) is one method of measuring the density of D. folliculorum and D. brevis present on a human host. One test suggests D. folliculorum is present in about 12% of human hair follicles. [2].
D. folliculorum tend to stay hidden in hair follicles during the day and move around only in darkness. Studies have indicated D. folliculorum have been living on humans almost since the dawn of our species, and are among the first organisms to visit the moon alongside their host astronauts.[3].
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Section 1 Genetics
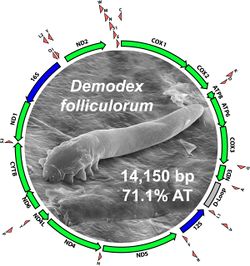
D. folliculorum belong to the Animalia kingdom, the Arthropoda phylum, the Chelicerata subphylum, the Arachnida class, the Acariformes superorder, the Trombidiformes order, the Eleutherengonides supercohort, and the Demodicae family of mites. Estimates of the divergence of D. folliculorum and D. brevis range from 87-173 million of years ago. The mitochondrial genome of D. folliculorum is 14,150 base pairs long and on average 71% made up of AT-pairs. The genome includes thirteen protein-encoding genes, two rRNA genes, and twenty-two tRNA genes. Its Genbank accession number is KM114226.[4].
The D. folliculorum genome is nearly the same size as that of D. brevis (14,240 bp) and within the average range of other Acariformes. A likely explanation is that mites in this superorder face similar selection pressures that favor a compact genome for easier replication. The high amount of AT-pairs also improves metabolic efficiency as it requires less H-bonds to be broken in the formation of a replication fork. D. folliculorum shares the same number of protein-encoding, rRNA, and tRNA genes as all other Acariformes; however, their unique arrangement is only shared by D. brevis. D. folliculorum possesses several other recognizable traits of Acariformes, such as the readiness of movement of tRNA genes and the truncated form of these genes. It is believed that D. brevis and D. folliculorum inherited the same tRNA structures from a common ancestor as the average sizes of their tRNA genes (53.5 bp in D. brevis and 53.3 in D. folliculorum) are very close. Truncated tRNA genes found in both species also share their lack of T-arms and D-arms. Due to the divergence of D. brevis and D. folliculorum occurring many millions of years ago, scientists believe that selection pressure is maintaining these truncated genes. It possible they perform a novel function or can be repaired in some way.[5].
The evolutionary history of D. folliculorum is closely intertwined with their host species: humans. A study of human hosts from various geographical locations revealed that 27% of the D. folliculorum genome can be segregated according to the geographic ancestry of their human hosts. Furthermore, 4 separate clades of D. folliculorum have been established which reflect the migration of humans out of Africa. People of African descent hosted the most diverse sample of D. folliculorum while people of Asian or European descent hosted one or two specific clades. People of Latin American descent also hosted diverse D. folliculorum, which can be explained by the African slave trade migrating millions of African people into the continent. [6].
The morphology of D. folliculorum shares many similarities to that of D. brevis. Adult D.folliculorum range from 0.3-0.4 mm in length and are composed of two fused body segments. Both segments are covered in scales and assist in anchoring to the hair follicle. The anterior segment has eight short legs attached that allow movement of 8-16 mm/h. Also on the anterior segment is the mouth with a palpus that is specialized for eating sebum, skin cells, and hormones. [7]. [8]. The posterior segment contains a genital opening in both sexes. Rounder and shorter female mites mate with males on the opening of the hair follicle and undergo internal fertilization. Females lay their eggs inside the hair follicle, which hatch into six-legged larvae after 3-4 days and reach adulthood in 7 days. [9]. The full lifespan of D. folliculorum is stretches to about 14-18 days.[10].
Section 2 Microbiome
D. folliculorum localize on the human face, feeding on skin cells, sebum, and hormones. They inhabit hair follicles, particularly those of the eyelashes and eyebrows. Several mites can inhabit the same follicle and will climb to the opening in order to mate or transfer hosts. Infants typically acquire D. folliculorum from physical contact with their mothers, however, the lack of sebum production in young children and adolescents prevents D. folliculorum from colonizing efficiently. As humans reach adulthood, sebum production increases substantially, peaking at ages 20-30. Therefore, although D. folliculorum are rarely found on children less than 5 years old, they are present on every adult that has been tested. D. folliculorum have been shown to colonize more on men than on women. Additionally, infestation tends to be the worst in the elderly and those with immunodeficiency. [11].
Conflicting sources characterize D. folliculorum as either commensals or parasites of their human hosts. In the majority of cases, the presence of D. folliculorum has no harmful effects on their hosts. However, in some cases infestation can lead to serious medical conditions such as acne rosacea and marginal blepharitis.[12]. Demidicosis or demodicidosis are blanket terms for diseases caused by Demodex mites. Demodicosis rosacea includes symptoms of “dryness, follicular scaling, superficial vesicles, and pustules.” [13]. The cause is tied to behaviors of the human host that encourage the mites’ replication such as neglecting to wash one’s face or using creamy/oily products. These behaviors lead to a build up of lipids, which D. folliculorum and D. brevis feed off of. [14]. In Blepharitis, bacteria carried by Demodex causes immune responses in the host. It includes symptoms of, “cylindrical dandruff, disorders of eyelashes, lid margin inflammation,” in addition to“itching, burning, foreign body sensation, crusting and redness of the lid margin, and blurry vision.”[15]. The inflammation induced by the bacteria may manifest in the conjuctiva or the cornea of the eyes, leading to further damage.[16]. Immunodeficiency has been shown to be a factor in the the infestation of D. folliculorum and D. brevis. [17].
Conclusion
Overall text length should be at least 1,000 words (before counting references), with at least 2 images. Include at least 5 references under Reference section.
References
- ↑ E. Yong. Everything you never wanted to know about the mites that eat, crawl, and have sex on your face. 2012. Discover Magazine. https://www.discovermagazine.com/planet-earth/ everything-you-never-wanted-to-know-about-the-mites-that-eat-crawl-and-have-sex-on-your-face
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ E. Yong. Everything you never wanted to know about the mites that eat, crawl, and have sex on your face. 2012. Discover Magazine. https://www.discovermagazine.com/planet-earth/ everything-you-never-wanted-to-know-about-the-mites-that-eat-crawl-and-have-sex-on-your-face
- ↑ M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.
- ↑ M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.
- ↑ M.F. Palopoli, D.J. Fergus, S. Minot, D.T. Pei, W.B. Simison, I. Fernandez-Silva, M.S. Thoemmes, R.R. Dunn, M. Trautwein. 2015. Global Divergence of the human follicle mite Demodex folliculorum: Persistent associations between host ancestry and mite lineages. Proceedings of the National Academy of Sciences of the United States of America. 112(52): 15958-15963.
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ E. Yong. Everything you never wanted to know about the mites that eat, crawl, and have sex on your face. 2012. Discover Magazine. https://www.discovermagazine.com/planet-earth/ everything-you-never-wanted-to-know-about-the-mites-that-eat-crawl-and-have-sex-on-your-face
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ J. Liu, H. Sheha, S.C.G Tseng. 2010. Pathogenic Role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 10(5): 505-510.
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ M.F. Palopoli, S. Minot, D. Pei, A. Satterly, J. Endrizzi. 2014. Complete mitochondrial genomes of the human follicle mites Demodex brevis and D. folliculorum: novel gene arrangement, truncated tRNA genes, and ancient divergence between species. BMC Genomics. 15(1): 1124.
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
- ↑ J. Liu, H. Sheha, S.C.G Tseng. 2010. Pathogenic Role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 10(5): 505-510.
- ↑ J. Liu, H. Sheha, S.C.G Tseng. 2010. Pathogenic Role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 10(5): 505-510.
- ↑ P. Rather, I. Hassan. 2014. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatology. 59(1): 20-66.
Edited by Scarlett Jones, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2019, Kenyon College.
