Desulfurobacterium: Difference between revisions
No edit summary |
|||
| (42 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<big><big><strong><var>Desulfurobacterium thermolithotrophum</var></strong></big></big> | |||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
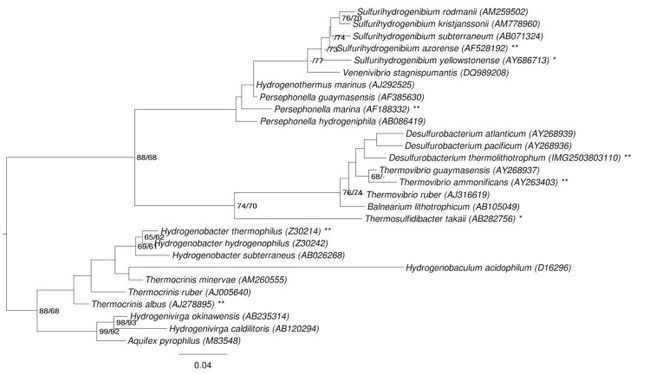
[[File:Phylogeny Tree.jpg|thumb|right|650px|<strong> Figure 1.</strong> Phylogenetic tree highlighting the position of <var>D. thermolithotrophum</var> (middle of figure) relative to the type strains of the other species within the order Aquificales (Göker et. al., 2011). ]] | |||
{| | |||
| height="10" bgcolor="#FFDF95" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=2&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy]''' | |||
|} | |||
Domain: Bacteria | Domain: Bacteria | ||
| Line 24: | Line 30: | ||
<var>Desulfurobacterium atlanticum, Desulfurobacterium crinifex, Desulfurobacterium pacificum</var> | <var>Desulfurobacterium atlanticum, Desulfurobacterium crinifex, Desulfurobacterium pacificum</var> | ||
==Description and Significance== | ==Description and Significance== | ||
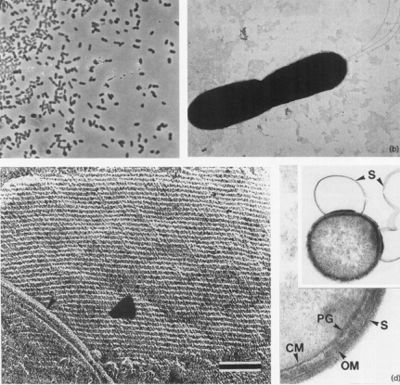
[[File:Desulfurobacterium thermolithotrophum Structure.jpg|thumb|right|400px| <strong>Figure | [[File:Desulfurobacterium thermolithotrophum Structure.jpg|thumb|right|400px| <strong>Figure 2.</strong> Phase-contrast (a) and electron micrographs of isolate BSA (b-d) , negatively stained dividing cell showing polar flagella (b), Freeze-etched cells and metal-shadowed intact cells of isolate BSA (c) showing the S-layer lattice, and typical cell envelope of a Gram-negative bacterium becoming visible by ultrathin selection (d) (L'Haridon et. al., 1998).]] | ||
<big><strong>Appearance:</strong></big><br> | <big><strong>Appearance:</strong></big><br> | ||
Cells appear as small rods, about 1-2 µm long and 0.4-0.5 µm wide (seen in Figure | Cells appear as small rods, about 1-2 µm long and 0.4-0.5 µm wide (seen in Figure 2a and b) and are stained as Gram-negative. These cells can occur either singly or in pairs, and are observed to be highly motile. Through negative staining, up to three flagella could be observed under a microscope. Moreover, during the stationary growth phase, some rods become spherical (L'Haridon et. al., 1998). | ||
| Line 44: | Line 45: | ||
Most extremely thermophilic microorganisms that are found in deep-sea hydrothermal vents are archaea species. However, <var>Desulfurobacterium thermolithotrophum</var> is the first bacteria capable of serving as a primary producer in such an environmental conditions (L'Haridon et. al., 1998). | Most extremely thermophilic microorganisms that are found in deep-sea hydrothermal vents are archaea species. However, <var>Desulfurobacterium thermolithotrophum</var> is the first bacteria capable of serving as a primary producer in such an environmental conditions (L'Haridon et. al., 1998). | ||
==Genome Structure== | |||
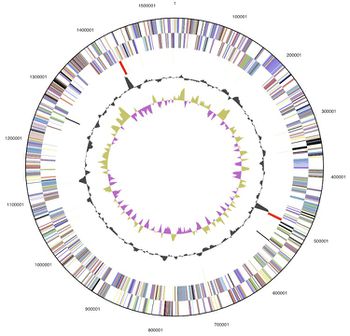
[[File:Desulfuro Genome.jpg|thumb|left|350px|<strong>Figure 3.</strong> Graphical circular map of the genome. From bottom to top: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, GC skew (Göker et. al., 2011).]] | |||
<strong><big>Genome Size:</big></strong><br> | |||
<var>D. thermolithotrophum</var> has one circular chromosome, with 1.54 Mb (or 1,541,968 base pairs long). It has 1,594 genes, with 1,543 protein-coding regions (96.8%) and a G+C Content of ~35%. 34 of the genes are also known to be pseudo genes, however 1,204 (75.53%) of the total genes have a predicted function (Göker et. al., 2011). | |||
<strong><big>Interesting Features:</big></strong><br> | |||
On the 16s rRNA sequence, between positions 198-219, there is a CUC bulge characteristic of the aquificales lineage (Göker et. al., 2011). The observation of this phenomenon has led to recent discrepancy as to which specific classification order the species should be included. | |||
==Cell Structure, Metabolism and Life Cycle== | |||
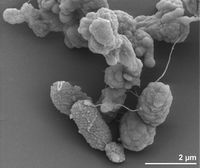
[[File:Desulfurobacterium thermolithotrophum Larger Structure.jpg|thumb|right|200px|<strong>Figure 4.</strong> Scanning electron micrograph of <var>D. thermolithotrophum</var> BSAT (Göker et. al., 2011).]] | |||
<big><strong>Cell Structure:</strong></big><br>The cells of <var>D. thermolithotrophum</var> are small and rod-shaped, ranging from about 1-2 micrometers long & 0.4-0.5 micrometers wide. However, some cells can become spherical during stationary growth phase (L'Haridon et. al., 1998). Some scientists have theorized that such changes are used to combat changing environmental conditions during nutrient uptake in order to keep maximum efficiency. In this aspect, small, spherical cells are observed to undergo diffusion at the most efficient rate (Young, 2006). Cells obtain nutrients for metabolic processes through the cell membrane. Therefore, in order to compensate for a larger amount of nutrients inside of the cell during growth, the cell must increase its surface area (Young, 2006). The cells of <var>D. thermolithotrophum</var> do so by developing the spherical shape, which is thought to allow them to maximize the volume within the cell while keeping the amount of exposed surface relatively unchanged (L'Haridon et. al., 1998). The cells are observed to occur in either singles or in pairs as highly motile (up to 3 flagella) and containing an oblique S-Layer lattice that frequently peels off to form loops. The observed peeling, according to Sleytr & Beveridge (1999), may serve to prevent the cells from clogging further envelope layers. These results ultimately suggest a high amount of lipids & lipoproteins due to the outer membrane present (L'Haridon et. al., 1998). | |||
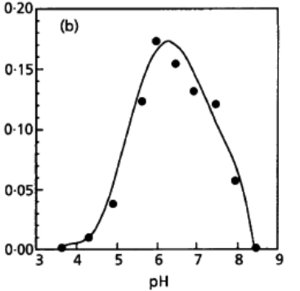
[[File:PH Growth Rate.png|thumb|right|300px|<strong>Figure 5.</strong> Growth rate in respective pH at Optical Density 600 (L'Haridon et. al., 1998).]] | |||
[[File: | |||
<strong> | <big><strong>Metabolism & Life Cycle:</strong></big><br><var>D. thermolithotrophum</var> are observed to be strict anaerobes, meaning that they do not utilize oxygen as an electron acceptor and are actually harmed by its presence in large amounts. Instead, these organisms use sulfur as its electron acceptor, as well as hydrogen gas (H2) as the electron donor in reducing sulfate into hydrogen sulfide gas. Aside from sulfur, these organisms can also utilize: thiosulfate, sulfite, and other polysulfides as alternative electron acceptors. However, <var>D. thermolithotrophum</var> were unable to use cysteine, sulphate, nitrate, or nitrite in this process (L'Haridon et. al., 1998). These organisms are classified as chemolithoautotrophs, due to their utilization of energy from reduced mineral compounds (such as rock sediments or elemental sulfur), along with chemical reactions occurring between carbon dioxide and hydrogen gas available, to behave as primary producers (autotrophs) in their ecosystem. | ||
==Ecology and Pathogenesis== | |||
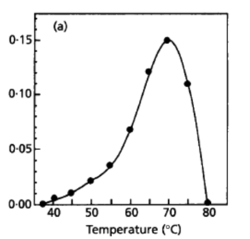
[[File:Temperature Optimal.png|thumb|left|250px|<strong>Figure 6.</strong> Growth Rate at respective Temperature at Optical Density 600 (L'Haridon et. al., 1998).]] | |||
<big><strong>Ecology:</strong></big><br> | |||
As stated above, <var>Desulfurobacterium thermolithotrophum</var> organisms were first collected in a deep sea hydrothermal vent, the Snake Pit Vent Field, in the Mid Atlantic Ridge. Though symbiotic interactions have not been observed due to lack of experimental research done for this organism, <var>D. thermolithotrophum</var> has a rare contribution to the environment in its ability to reduce (as opposed to oxidize) sulfur in its metabolic processes, which helps transfer energy from its geothermal source to higher trophic levels. Thus, scientists have hypothesized that these organisms may have mild symbiotic interactions with various invertebrates living in the oxygenated mixing area of the ocean, just above the highly reduced hydrothermal areas (Sievert & Kiene, 2007). | |||
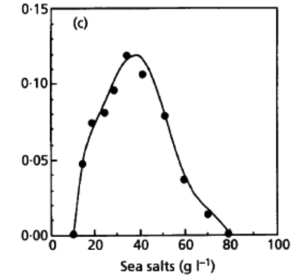
[[File:Salt Concentration.png|thumb|right|300px|<strong>Figure 7.</strong> Growth Rate in respective Salt Concentration at Optical Density 600 (L'Haridon et. al., 1998).]] | |||
<big><strong>Pathogenesis:</strong></big><br> | |||
No observations of disease causation, as a result of this organism, has been recorded. However, they are observed to have inhibited growth in the presence of antibiotics such as amphenicol, penicillin G, and rifampicin if introduced prior to incubation at optimal temperature (L'Haridon et. al., 1998). | |||
==References== | ==References== | ||
| Line 70: | Line 79: | ||
2. Göker M, Daligault H, Mwirichia R, et al. Complete genome sequence of the thermophilic sulfur-reducer <var>Desulfurobacterium thermolithotrophum</var> type strain (BSAT) from a deep-sea hydrothermal vent. <var>Standards in Genomic Sciences</var>. 2011;5(3):407-415. PubMed | 2. Göker M, Daligault H, Mwirichia R, et al. Complete genome sequence of the thermophilic sulfur-reducer <var>Desulfurobacterium thermolithotrophum</var> type strain (BSAT) from a deep-sea hydrothermal vent. <var>Standards in Genomic Sciences</var>. 2011;5(3):407-415. PubMed | ||
3. Sievert S M, Kiene R P, Schulz-Vogt H N. The Sulfur Cycle. <var>Oceanography</var>. 2007; 20(2):117-123. | |||
4. Sleytr U. B., Beveridge T. J.Bacterial S-layers. <var>Trends Microbiol.</var> 1999; 7:253–260. | |||
5. Young KD. The Selective Value of Bacterial Shape. <var>Microbiology and Molecular Biology Reviews.</var> 2006; 70(3):660-703. | |||
==Author== | ==Author== | ||
Page authored by William Van Cleef III & Meghan Von Holt, students of Professor Jay Lennon at Indiana University Bloomington. | Page authored by William Van Cleef III & Meghan Von Holt, students of Professor Jay Lennon at Indiana University Bloomington. | ||
[[Category:Pages edited by students of Jay Lennon at Indiana University]] | |||
Latest revision as of 03:58, 4 May 2015
Desulfurobacterium thermolithotrophum
Classification
|
NCBI: Taxonomy |
Domain: Bacteria
Phylum: Aquificae
Class: Aquificae
Order: Desulfurobacteriales
Family: Desulfurobacteriaceae
Genus: Desulfurobacterium
Species
Desulfurobacterium thermolithotrophum
Related Species:
Desulfurobacterium atlanticum, Desulfurobacterium crinifex, Desulfurobacterium pacificum
Description and Significance
Appearance:
Cells appear as small rods, about 1-2 µm long and 0.4-0.5 µm wide (seen in Figure 2a and b) and are stained as Gram-negative. These cells can occur either singly or in pairs, and are observed to be highly motile. Through negative staining, up to three flagella could be observed under a microscope. Moreover, during the stationary growth phase, some rods become spherical (L'Haridon et. al., 1998).
Habitat:
Desulfurobacterium thermolithotrophum are anaerobic chemolithoautotrophs, typically found in hot, deep-sea hydrothermal vents, such as the Snake Pit vent field of the Mid-Atlantic Ridge. These thermophiles are capable of survival in temperatures ranging from 40°-75°C, but prefer an optimal temperature of 70°C. In addition to a tolerance to a variety of temperatures, this strain has also been observed (in vitro) to survive in conditions ranging from a pH of 4.4-8.0, with an optimal pH level of 6.0. A medium of salt concentration 35 g/L would be most preferred by this species for cultivation, yet a range of 15-70 g/L would also be suitable for growth. Given these optimal conditions are met, the experimentally observed doubling time was ~135 minutes (L'Haridon et. al., 1998).
Significance:
Most extremely thermophilic microorganisms that are found in deep-sea hydrothermal vents are archaea species. However, Desulfurobacterium thermolithotrophum is the first bacteria capable of serving as a primary producer in such an environmental conditions (L'Haridon et. al., 1998).
Genome Structure
Genome Size:
D. thermolithotrophum has one circular chromosome, with 1.54 Mb (or 1,541,968 base pairs long). It has 1,594 genes, with 1,543 protein-coding regions (96.8%) and a G+C Content of ~35%. 34 of the genes are also known to be pseudo genes, however 1,204 (75.53%) of the total genes have a predicted function (Göker et. al., 2011).
Interesting Features:
On the 16s rRNA sequence, between positions 198-219, there is a CUC bulge characteristic of the aquificales lineage (Göker et. al., 2011). The observation of this phenomenon has led to recent discrepancy as to which specific classification order the species should be included.
Cell Structure, Metabolism and Life Cycle
Cell Structure:
The cells of D. thermolithotrophum are small and rod-shaped, ranging from about 1-2 micrometers long & 0.4-0.5 micrometers wide. However, some cells can become spherical during stationary growth phase (L'Haridon et. al., 1998). Some scientists have theorized that such changes are used to combat changing environmental conditions during nutrient uptake in order to keep maximum efficiency. In this aspect, small, spherical cells are observed to undergo diffusion at the most efficient rate (Young, 2006). Cells obtain nutrients for metabolic processes through the cell membrane. Therefore, in order to compensate for a larger amount of nutrients inside of the cell during growth, the cell must increase its surface area (Young, 2006). The cells of D. thermolithotrophum do so by developing the spherical shape, which is thought to allow them to maximize the volume within the cell while keeping the amount of exposed surface relatively unchanged (L'Haridon et. al., 1998). The cells are observed to occur in either singles or in pairs as highly motile (up to 3 flagella) and containing an oblique S-Layer lattice that frequently peels off to form loops. The observed peeling, according to Sleytr & Beveridge (1999), may serve to prevent the cells from clogging further envelope layers. These results ultimately suggest a high amount of lipids & lipoproteins due to the outer membrane present (L'Haridon et. al., 1998).
Metabolism & Life Cycle:
D. thermolithotrophum are observed to be strict anaerobes, meaning that they do not utilize oxygen as an electron acceptor and are actually harmed by its presence in large amounts. Instead, these organisms use sulfur as its electron acceptor, as well as hydrogen gas (H2) as the electron donor in reducing sulfate into hydrogen sulfide gas. Aside from sulfur, these organisms can also utilize: thiosulfate, sulfite, and other polysulfides as alternative electron acceptors. However, D. thermolithotrophum were unable to use cysteine, sulphate, nitrate, or nitrite in this process (L'Haridon et. al., 1998). These organisms are classified as chemolithoautotrophs, due to their utilization of energy from reduced mineral compounds (such as rock sediments or elemental sulfur), along with chemical reactions occurring between carbon dioxide and hydrogen gas available, to behave as primary producers (autotrophs) in their ecosystem.
Ecology and Pathogenesis
Ecology:
As stated above, Desulfurobacterium thermolithotrophum organisms were first collected in a deep sea hydrothermal vent, the Snake Pit Vent Field, in the Mid Atlantic Ridge. Though symbiotic interactions have not been observed due to lack of experimental research done for this organism, D. thermolithotrophum has a rare contribution to the environment in its ability to reduce (as opposed to oxidize) sulfur in its metabolic processes, which helps transfer energy from its geothermal source to higher trophic levels. Thus, scientists have hypothesized that these organisms may have mild symbiotic interactions with various invertebrates living in the oxygenated mixing area of the ocean, just above the highly reduced hydrothermal areas (Sievert & Kiene, 2007).
Pathogenesis:
No observations of disease causation, as a result of this organism, has been recorded. However, they are observed to have inhibited growth in the presence of antibiotics such as amphenicol, penicillin G, and rifampicin if introduced prior to incubation at optimal temperature (L'Haridon et. al., 1998).
References
1. L'Haridon S, Cilia V, Messner P, Raguénès G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. International Journal of Systematic Bacteriology , 1998; 48:701-711. PubMed
2. Göker M, Daligault H, Mwirichia R, et al. Complete genome sequence of the thermophilic sulfur-reducer Desulfurobacterium thermolithotrophum type strain (BSAT) from a deep-sea hydrothermal vent. Standards in Genomic Sciences. 2011;5(3):407-415. PubMed
3. Sievert S M, Kiene R P, Schulz-Vogt H N. The Sulfur Cycle. Oceanography. 2007; 20(2):117-123.
4. Sleytr U. B., Beveridge T. J.Bacterial S-layers. Trends Microbiol. 1999; 7:253–260.
5. Young KD. The Selective Value of Bacterial Shape. Microbiology and Molecular Biology Reviews. 2006; 70(3):660-703.
Author
Page authored by William Van Cleef III & Meghan Von Holt, students of Professor Jay Lennon at Indiana University Bloomington.