Ear: Difference between revisions
No edit summary |
|||
| (86 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
==Intro to the Ear== | ==Intro to the Ear== | ||
[[Image:The_Ear_-_Image.gif|right|400x]] | [[Image:The_Ear_-_Image.gif|right|400x|frame|none|Image of the outer, middle, and inner ear. Image taken with permission from Brittanica. [[http://www.britannica.com/EBchecked/topic/175622/ear#assembly=url~http%3A%2F%2Fwww.britannica.com%2FEBchecked%2Ftopic-art%2F175622%2F530%2FStructure-of-the-human-ear]]]] | ||
The human ear is comprised of 3 main parts: outer, middle, and inner. | |||
The outer ear consists of the pinna | The outer ear consists of the pinna(the visible part of the ear) and the auditory ear canal to the tympanic membrane(ear drum). The membrane separates the outer ear from the middle ear which is an air-filled space that is connected to the nasopharynx via the eustachian tube. The middle ear consists of the malleus, incus, and stapes. These three tiny bones carry sound from the ear drum to the inner ear. The inner ear, also known as the bony labyrinth, is a fluid filled compartment that surrounds the membraneous labyrinth which encloses both the cochlea and the vestibular apparatus.(1) | ||
===Physical Conditions of the Normal Healthy Ear=== | ===Physical Conditions of the Normal Healthy Ear=== | ||
The conditions of the pinna are similar to that of the [[skin]]. Closer to the ear canal the skin develops a thin lining of ear wax. Ear wax is composed mostly of dead skin cells and keratin with a small mixture of cerumen, sweat, and oil. Cerumen is secreted from the ceruminous glands located in the first third outer part of the ear canal and is thought to be composed mainly of cholesterol, squalene, wax esters, ceramides, and triglycerides. The cerumen also has | The conditions of the pinna are similar to that of the [[skin]]. Closer to the ear canal the skin develops a thin lining of ear wax. Ear wax is composed mostly of dead skin cells and keratin with a small mixture of cerumen, sweat, and oil. Cerumen is secreted from the ceruminous glands located in the first third outer part of the ear canal and is thought to be composed mainly of cholesterol, squalene, wax esters, ceramides, and triglycerides. The cerumen also has antimicrobial properties which can be attributed to its slight acidic pH of 5 and the presence of lysozyme. In normal circumstances, the ear wax is continuously pushed out of the ear canal by the slow migration of the top layer of skin cells from the tympanic membrane towards the outer ear. The ear wax traps any foreign particles and organisms on its way out. Ear wax type also varies between people. Generally Native American and Southeast Asians present a dry flaky type of ear wax where as Europeans and Africans produce the "wet type." The dry type falls out easier and is in thinner concentration while the wet type is thicker and stickier, though it should readily fall out as well under normal circumstances. The ear canal is warm(~37C), moist, and abundant with nutrients from dead skin cells. Current research on ear wax shows that some of the antimicrobial properties of the cerumen can be attributed to the presence of antimicrobial peptides, Human Beta-defensin 1 and Human Beta-defensin 2. Other recent studies have shown that cerumen directly inhibits the growth of <i>Staphylococcus aureus</i>, <i>Pseudomonas aeruginosa</i>, and <i>Candida albicans</i>, but its effect on the growth of <i>E. coli</I> remains to be determined (22)(5)(35). In the absence of cerumen, the ear canal would be an optimal environment for microbes. | ||
The middle ear, also known as the tympanic cavity, is an air filled space that is seperated from the outer ear by the tympanic membrane and from the inner ear by the fenestra vestibuli. A mucous membrane lines the middle ear that is continuous with the nasal passageways and nasopharynx via the eustachian tube. This continuity allows the person to equalize the air pressure inside the cavity with that of the outside environment. Mucus has long been known to contain many antimicrobial enzymes and immunoglobulins. In addition to its anti microbial properties, the mucus also serves to trap any foreign particles and microbes that are introduced into the middle ear. Ciliary action of the membrane promptly flushes them out to the nasopharynx. This migration of mucus is important to prevent microbes from colonizing as the tympanic cavity would otherwise present a very favorable environment for microbes. It is moist, abundant in nutrients, protected from the external environment, and at body temperature. (9)(30) | |||
The inner ear consists of two labyrinth structures. The bony labyrinth and the membraneous labyrinth. The bony labyrinth is filled with a fluid called perilymph and encloses the membraneous labyrinth (13). The membraneous labyrinth comprises the vestibular apparatus and the cochlea. It is filled with a seperate fluid that does not mix with the perilymph called endolymph. Both fluids are segregated but derived from blood plasma implying a high concentration of nutrients and a similar pH of ~7.4. In addition to nutrients, small concentrations of immunoglobulins present in blood serum are also found in the perylimph and endolymph, filtered through by the blood-labyrinth barrier (15). A characteristic of perilymph is that its concentration of sodium is high (about 150 milliequivalents per litre) and its concentration of potassium is low (about 5 milliequivalents per litre), similar to other extracellular fluids. Conversely endolymph has a low concentration of sodium (about 15 milliequivalents per litre) and a high concentration of potassium (about 140 milliequivalents per litre) (1). Since the inner ear is completely membrane enclosed, it should be virtually free of bacteria under normal circumstances. | |||
The inner ear consists of two labyrinth structures. The bony labyrinth and the membraneous labyrinth. The bony labyrinth is filled with a fluid called perilymph and encloses the membraneous labyrinth. The membraneous labyrinth comprises the vestibular apparatus and the cochlea. It is filled with a seperate fluid that does not mix with the perilymph called endolymph. | |||
===Microbes in the Healthy Ear=== | ===Microbes in the Healthy Ear=== | ||
Because it is exposed to the outside environment, despite the best efforts of the ceruminous glands, the | Because it is exposed to the outside environment, despite the best efforts of the ceruminous glands, the healthy outer ear still houses a variety of microbes. Some of the most common bacteria are <i>Staphylococcus epidermis, Turicellaotitidis, Alloiococousotitis, Pseudomonas aeruginosa, Corynebacterium, Staphylococcus aureus</I>, and <i>Streptococcus saprophyticum</I>. The most common fungal microbe known to reside in the ear is <i>Candida albicans</I> (29)(7). Under normal circumstances, this bacterial flora does not thrive. | ||
Microbes that are known to inhabit the middle ear are <i>Streptococci, Haemophilus pneumoniae, Moraxella catarrhalis</I>, and less commonly <i>Mycobacterium</i>. These bacteria are known to get to the middle ear by migrating through the nasophraynx and | Microbes that are known to inhabit the middle ear are <i>Streptococci, Haemophilus pneumoniae, Moraxella catarrhalis</I>, and less commonly <i>Mycobacterium</i>. These bacteria are known to get to the middle ear by migrating through the nasophraynx and Eustachian tube. Recent research shows that the presence of alpha hemolytic <i>Streptococci</I> from healthy children (as opposed to children with recurrent acute otitis media) inhibit the growth of pathogenic S. pneumoniae and H. influenzae. As a result of this research, there is now an interest in developing a nasal spray that deposits alpha hemolytic <i>Streptococci</i> into the nasopharynx as a means to help prevent rAOM (32). | ||
Because of the blood-labyrinth barrier and the presence of immunoglobulins in the inner ear fluids there should be virtually no microbes living normally in the inner ear. | |||
===Normal Changes to the Environment of the Ear=== | ===Normal Changes to the Environment of the Ear=== | ||
The conditions of the outer ear are subject to the most variation as it is exposed to the outside environment. One of the biggest effects on the environment of the ear would be those that remove the ear wax. This can occur through excessive showering or swimming as water or soap can wash away the thin protective lining of ear wax. Loss of the cerumen encourages the growth of microbes that are normally kept under control by its antimicrobial properties. Use of cotton swaps or hair pins is also common practice as a means to remove ear wax. In addition to the removal of the ear wax, they can also scratch the surface of the skin which can allow microbes to get in to the unprotected tissue. Use of cotton swabs can also push the ear wax further into the canal, which if allowed to build up can become impacted and damage the inner ear canal and the tympanic membrane. These too provide opportunities for microbes to infect the tissue. | |||
Opportunities for microbes to infect the ear diminish as the person ages. Young children with immature immune systems are more susceptible to infection by microbes than are adults with developed immune systems. | |||
Blockage of the Eustachian tube can also increase the likelihood of ear infections. This occurs when the mucus in the tube gets too thick or becomes blocked, as can occur during infections or allergic reactions of the upper respiratory tract. The opening of the Eustachian tube is also affected by head positioning. A head elevation of 20 degrees above horizontal (as can occur while lying down with a pillow) reduces the flow of air to two thirds of normal while a complete horizontal position reduces it to one third. Blockage of the tube prevents a healthy flow of mucus out of the middle ear and as a result, microbes present inside the middle ear become trapped and are allowed to proliferate.(30) | |||
Blockage of the Eustachian tube can also increase the likelihood of ear infections. This occurs when the mucus in the tube gets too thick or becomes blocked as can occur during infections or allergic reactions of the upper respiratory tract. The opening of the Eustachian tube is also affected by head positioning. A head elevation of 20 degrees above horizontal (as can occur while lying down with a pillow) reduces the flow of air to two thirds of normal while a complete horizontal position reduces it to one third. Blockage of the tube prevents a healthy flow of mucus out of the middle ear and as a result, microbes present inside the middle ear become trapped and are allowed to proliferate. | |||
==Infections of the Ear== | ==Infections of the Ear== | ||
===Outer Ear=== | |||
<b>Auditory Ear Canal</b> | |||
[[Image:P.aeruginosa_-_11.jpg |right|100x|frame|none|An image of the bacteria, <i> Pseudomonas aeruginosa</i>, known to cause infections of the outer ear. [[http://www.gasdetection.com/news2/Pseudomonas_aeruginosa.jpg]]]] | |||
Even though the human ears are exposed to environment conditions they get rarely infected; one can nearly always bathe, swim, and walk in the rain without any problems. This is because the auditory canal is protected by a thin lining of ear wax and the shape of the ear which serves to tip out any fluids (22). Acute external otitis, also known as Swimmer's Ear, is an infection that takes place in the outer auditory canal. Constant exposure to water increases the humidity in the ear. The excess moisture weakens the ear’s external skin and reduces the amount of protective waxes. External otitis can also be caused by swimming in polluted water, scratching the ear or inside the ear, or if an object gets stuck in the ear (2). In these circumstances, bacteria are able to grow and infect the ear canal since they love to live in areas in warm and humid conditions. Even though the outer ear is not as prone to infection as some other parts of the human body it will become infected if, for example, one swims in unsanitized water. Residual dirt and oil from previous swimmers serve as nutrients for organisms like <i>Pseudomonas aeruginosa</i>, the main cause of swimmer's ear, which have simple nutritional requirements. From the water, <I>Pseudomonas aeruginosa</I> is able to swim fast to the ear with its polar flagella. It also uses quorum sensing to detect the density of immediate population which allows them to form biofilms on inanimate surfaces. This bacterium likes to live in aerobic environments; thus, the outer ear is an ideal habitat for this type of organism. This gram negative develops a protective exopolysaccharide coat in biofilm. This gives the bacterium resistance to many antibacterial compounds and antiseptics. It also allows the bacteria to survive harsh conditions including fluctuating temperatures and high salt concentrations (34). The only antibiotics that were found to have some effect on <i>Pseudomonas aeruginosa</i> are gentamicin, imipenem, and fluoroquinolones(34). | |||
Less common bacterial causes of swimmer’s ear are <I>Staphylococcus aureus, Proteus mirabilis, Streptococci species</I>, and various gram negative <i>Bacilli</i>. Fungal infections, caused by <I>Aspergillus niger</I> or<I> Candida albicans</I>, are even rarer. According to the current research article, “<I>Staphylococcus aureus</I> Serves as an Iron Source for <I>Pseudomonas aeruginosa</I>a during In Vivo Coculture,” states that <i>pseudomonas aeruginosa</i> uses lysed <i>staphylococcus aureus</i> to get its required amount of iron (21). However, it is still not known if the <I>Pseudonomas aeruginosa</I>a induces the lysing or if <I>Staphylococcus aureus</I> auto lyses. | |||
<b>Ear Piercings</b> | |||
The micro organisms <I>Pseudomonas aeruginosa</I> and <I>Staphylococcus</I> are both involved in the cause of sporadic cartilage infections following ear piercing or other trauma. <I>Pseudomonas aeruginosa</I> is a gram-negative, free-living aerobic bacterium belonging to the bacterial family <I>Pseudomonadaceae</I>. It is commonly found in water and soil and is known to infect human tissues. <I>Pseudomonas aeruginosa</I> is an opportunistic pathogen. This bacterium almost never infects healthy tissues. But if tissue defenses are compromised in some manner, there is hardly any tissue that it cannot infect. Risk for infections increase when newly pierced ears are exposed to <I>Pseudomonas</I> organisms present in lakes or any stagnant water. One way against the activity of these serious infectious organisms is to expose them to the newly discovered fluoroquinoline antibiotic, ciprofloxacin. Ciprofloxacin offers the most antipseudomonal activity against <I>Pseudomonas aeruginosa</I> in vitro. This class of antimicrobial agent also has good activity against <I>staphylococcal</I> species, but resistance can be developed in patients with repeated or prolonged use (28)(29). | |||
<I> | |||
===Middle Ear=== | ===Middle Ear=== | ||
[[Image:Haemo_influ.jpg|right|100x|frame|none|An image of the bacteria, <i>Haemophilus influenzae</i>, known to be the main cause of otitis media in the middle ear. [[http://www.microbelibrary.org/microbelibrary/files/ccImages/Articleimages/Buxton/03%20Spt%20neg%20diplo/Haemophilus%20influenzae%20fig1.jpg]]]] | |||
Otitis media is an infection of the middle ear, caused mainly by <i>Haemophilus influenza, Streptocococcus pneumonia</i>, or <i>Moraxella catarrhalis</i>, that mostly infects children at a young age. It is harder for young children to fight the microbe or a bacterium because their immune system is not fully developed. Ear infections are often associated with dysfunction or swelling of the Eustachian tubes. The Eustachian tube is the principal portal for the entry and exit of bacterial in the middle ear. During a viral upper respiratory tract infection(i.e. the common cold), inflammation can block the Eustachian tube. As a result the middle ear does not drain properly and the fluid builds up, becoming a breeding ground for bacteria. Additionally, children have larger adenoid glands than adults. These glands are located behind the throat by the Eustachian tubes and are involved in lymphocyte production. During infections they swell and can block the Eustachian tubes. Furthermore, the adenoid glands themselves can become infected and this infection can directly spread to the tubes (30)(11). | |||
[[Image:Moraxella_catarrhali.jpg|left|100x|frame|none|<i> Moraxella catarrhalis</i>. A less common bacteria known to cause otitis media]] | |||
Moraxella catarrhalis | The most common bacteria that grows in the middle ear and causes Otitis media is <i>Haemophilus Influenzae</i> (11). The <i>Haemophilus</I> bacterium grows optimatlly at 35-37C(human body temp.), at a pH of 7.6, and prefers nutrients that can be found in the blood, such as "hemin, NAD or NADP" (33). Based on a recent research article, "Histidine auxotrophy in commensal and disease-causing nontypeable <i>Haemophilus influenza</I>," the lack of histidine is a limiting factor in the growth of bacteria in the middle ear. Because histidine is found in low concentrations in the middle ear, strains of <i>Haimophilus</i> that are predominantly associated with otitis media are highly conserved for an operon encoding histidine producing enzymes (16). Recent studies of <i>Haemophilus influenzae</I> have shown that it can take advantage of the host's immune systems to promote its survival. In a petri dish, it has been shown that <i>Streptococcus pneumoniae</I> out competes <i>Haemophilus</i> by producing peroxides that interfere with its membrane molecules. However, within the respiratory tract of the host, <i>Haemophilus</i> is able to induce an immune response that causes the host to respond only to <i>Streptococcus</i>, thus elminating the competition (18). | ||
<I>Moraxella catarrhalis</I> is a gram-negative and oxidase-positive diplococcus which lives in an aerobic environment and is found only in humans. <I>Moraxella catarrhalis</I> is a mucosal pathogen which commonly causes otitis media in young children (8). Its strains can tolerate lower temperature and can grow well at 28ºC(25). Iron plays an important role in helping most microbes survive. However, most of the iron in the human body is coupled to carrier proteins such as transferrin (serum) and lactoferrin (mucosal surface). In order for this bacterium to grow under these limited-iron conditions, <I>Moraxella catarrhalis</I> has to compete for iron by directly utilizing human carrier proteins without any siderophore production (6). This mechanism is similar to <I>H. influenzae</I> and <I>Neisseria</I> species which allow these mucousal pathogens to maintain their growth. With this advantageous feature of being able to utilize these serum carrier proteins, iron-limited <I>M. Catarrhalis</I> can colonize and survive on human mucosal surfaces, as in the middle ear. Prior to otitis media, <I>Moraxella catarrhalis</I> attaches to the respiratory via Type IV pili (TFP). TFP also plays an important part in biofilm formation. From there it migrates to the middle ear via the Eustachian tube to initiate infection (20)(24). | |||
Streptococcus pneumoniae is another bacterium that can cause otitis media. Currently there is a study to prevent the child ear infection through the use of a nasal spray. A study on mice Miranda Hitti's lab indicates that the protein lysin can be used to attack Streptococcus pneumonia. Mice infected with Streptococcus pneumoniae and administered the lysin nasal spray did not develop infections where as 80% of the mice that received a placebo did (12). | |||
===Inner Ear=== | ===Inner Ear=== | ||
Inner ear infections can be caused by either bacterial or viral infections. Since the inner ear is enclosed and | [[Image:Streptococcus pneumoniae 2 - image.gif|right|40x|frame|none|Image of <i>Streptococcus pneumonia</i>, a common pathogen found to infect the inner ear. [[http://mic.sgmjournals.org/content/vol152/issue2/images/medium/frontcover.gif]]]] | ||
Inner ear infections can be caused by either bacterial or viral infections (14). Since the inner ear is enclosed and is in the deepest part of the ear, it is hard for bacteria to directly affect this area. Bacterial infections of the inner ear arise as a secondary effect of otitis media or bacterial meningitis (an inflammation in the lining of the brain) (14). Sensorineural hearing loss (SNHL) is a critical inner ear complications due to bacterial meningitis. Hearing loss can either occur by direct bacterial invasion or through the passage of bacterial toxins and inflammatory mediators into the inner ear. <I>Streptococcus pneumoniae</I> is one of the most frequently found bacteria to be associated with hearing loss (23)(3). | |||
S. pneumoniae is an extracellular bacterial pathogen enclosed by a capsule. It requires adherence to host cells to gain access to areas where it can initiate infection. | <I>S. pneumoniae</I> is an extracellular bacterial pathogen enclosed by a capsule. It requires adherence to host cells to gain access to areas where it can initiate infection (26). Normally <I>S. pneunomiae</I> resides in certain areas of the human body without being infectious. However, colonization of this bacterium can become infectious if the organism reaches the auditory tube. <I>S. pneumoniae</I> travels through the blood stream reaching the meninges located around the brain, where it can cause meningitis. They then travel from the cerebrospinal fluid and make their way into membranous labyrinth through the internal auditory canal or the cochlear aqueduct. The membranous labyrinth consists of the vestibule, the semicircular canals, and the cochlea. <I>S. pneumoniae</I> enters the inner ear and initiates ossification, filling the lumen of the cochlea and semicircular canals (27)(10). | ||
Knowledge of the relationship between hearing loss and bacterial meningitis is primarily based on clinical studies. However, many issues revolving this relationship, such as how the bacteria enter the inner ear and their time course for causing hearing loss are still unknown. Length of how long it takes for S. pneumoniae to infect the labyrinth is still uncertain. However, studies done on | Knowledge of the relationship between hearing loss and bacterial meningitis is primarily based on clinical studies. However, many issues revolving this relationship, such as how the bacteria enter the inner ear and their time course for causing hearing loss are still unknown. Length of how long it takes for <I>S. pneumoniae</I> to infect the labyrinth is still uncertain. However, studies done on mice show progressive loss of hearing was identified after 48 hours of exposure to <I>Streptococcus pneumoniae</I> (19)(4). | ||
Viruses have also been known to cause most inner ear attacks, but are usually able to resolve on their own. Vestibular neuritis, the technical term for dizziness, is an inner ear disease caused by a virus. The cause for many inner ear conditions are still unknown. However, current research have shown that certain viruses maybe responsible for infections of specific structures of the inner ear. Herpes simplex virus has been shown to have a link to sudden sensorineural hearing loss. In experiments done these viruses have infected primarily the sensory cells of the labyrinth. Mumps virus have infected principally endolymphatic structures. Influenza virus have shown to infect mesenchymal cells of the perilymphatic channels of the cochlea. These experiments show the possible causality of infections in the inner ear but studies are still being done (19). | |||
==References== | |||
1) “Anatomy of the Human Ear." Encyclopedia Britannica. 25 Aug. 2008<http://www.britannica.com/EBchecked/topic/175622/ear/65043/Endolymph-and-perilymph#tab=active~checked%2Citems~checked&title=human%20ear%20%3A%3A%20Endolymph%20and%20perilymph%20--%20Britannica%20Online%20Encyclopedia> | |||
2) Badshah, Cyrus. "Medical Encyclopedia." Medline Plus. 22 June 2007. 24 Aug. 2008 | |||
<http://www.nlm.nih.gov/medlineplus/ency/article/000622.htm> | |||
3) Bhatt, Samir, Chris Halpin, Wen Hsu, Britt A. Thedinger, Robert A. Levine, Elaine Tuomanen, and Joseph B. Nadol. "Hearing Loss and Pneumococcal Meningitis: An Animal Model." Laryngoscope 101 (1991): 1285-292. | |||
4) Boston, Mark E., and Barry Strasnick. "Inner Ear, Labyrinthitis." Emedicine. 6 Mar. 2008. 25 Aug. 2008 <http://www.emedicine.com/ent/topic226.htm>. | |||
5) Bortz JT, Wertz PW, Downing DT. "Composition of cerumen lipids." J Am Acad Dermatol. 1990 Nov;23(5 Pt 1):845-9. | |||
6) Campagnari, A A, K L Shanks, and D W Dyer. "Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production." Infection and Immunity 62 (1994): 4909-914. | |||
7) Campos A, Arias A, Betancor L, Rodríguez C, Hernández AM, López Aguado D, Sierra A. "Study of common aerobic flora of human cerumen." J Laryngol Otol. 112 (1998 ):613-6 | |||
8) Constantinescu, Michael. "Excerpt from Moraxella Catarrhalis Infections." Emedicine. 2 July 2008. 26 Aug. 2008 <http://www.emedicine.com/med/topic1500.htm>. | |||
9) Crouch, James. "Functional Human Anatomy" Lea and Febiger 1978. | |||
10) Dichgans, M., L. Jager, T. Mayer, K. Schorn, and H. Pfister. "Bacterial Meningitis in Adults." Neurology. 28 Nov. 1998. University of California San Diego. 24 Aug. 2008 <http://www.neurology.org/cgi/content/full/52/5/1003>. | |||
11) Haemophilus influenzae type b. The Pink Book: Revisions to 10th Edition, | |||
Epidemiology and Prevention of Vaccine Preventable Diseases. March 2008. <http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hib.pdf>. | |||
12) Hitti, Miranda. "Nasal Spray May Prevent Ear Infection." WebMD. 23 Mar. 2007. 27 Aug. 2008 <http://www.webmd.com/cold-and-flu/ear-infection/news/20070323/nasal-spray-may-prevent-ear-infection>. | |||
13) "Inner Ear Fluid Imbalance." Loyola Medicine. 26 Aug. 2008 <http://www.meddean.luc.edu/depts/otolaryn/patient_ed/pdf/ent%20inner%20ear%20fluid%20imbalance.pdf>. | |||
14) "Inner-ear infection-Labyrinthitis." Formula Medical Group. 24 Aug. 2008 <http://www.formulamedical.com/topics/head&neck/inner%20ear%20infection%20labyrinthitis.htm>. | |||
15) Joel M. Bernstein, Pearay L. Ogra. "Immunology of the Ear" Raven Press New York 1987 | |||
http://www. | 16) Juliao PC, Marrs CF, Xie J, Gilsdorf JR. "Histidine auxotrophy in commensal and | ||
disease-causing nontypeable Haemophilus influenza." Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA. 11 May 2007. 28 Aug. 2008 | |||
<http://www.ncbi.nlm.nih.gov/pubmed/17496076?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum>. | |||
18) Lysenko E, Ratner A, Nelson A, Weiser J (2005). "The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces". PLoS Pathog 1 (1): e1. doi:10.1371/journal.ppat.0010001 | |||
19) Le, Davis, and Johnson RT. "Experimental viral infections of the inner ear.I.Acute infections of the newborn hamster labyrinth." Lab Investigation 34 (1976): 349-56. | |||
20) Luke, Nicole R., Joseph A. Jurcisek, and Lauren O. Bakaletz. "Contribution of Moraxella catarrhalis Type IV Pili to Nasopharyngeal Colonization and Biofilm Formation." Infection and Immunity 17 (2007): 5559-564. | |||
21) Mashburn, Lauren M., Amy M. Jett, Darrin R. Akins, and Marvin Whiteley."Staphylococcus aureus Serves as an Iron Source for Pseudomonas aeruginosa during In Vivo Coculture." Journal of bacteriology 187 (1994): 554-66. | |||
22) Mayo Clinic Staff. "Swimmer's ear." Mayo clinic.com. 16 Oct. 2006. 24 Aug. 2008<http://www.mayoclinic.com/health/swimmers-ear/DS00473> | |||
23) Miller, Martha L., Aditya H. Gaur, and Ashir Kumar. "Meningitis, Bacterial." Emedicine. 4 Jan. 2008. 25 Aug. 2008 <http://www.emedicine.com/ped/topic198.htm>. | |||
24) "Moraxella Catarrhalis Antigen, Corresponding Gene and Uses Thereof." WIPO. 23 Aug. 2008 <http://www.wipo.int/pctdb/en/wo.jsp?ia=wo2001019996&display=desc>. | |||
25) "Moraxella catarrhalis." Centers for Disease Control and Prevention. 27 Aug. 2008 <http://www.cdc.gov/std/gonorrhea/lab/mcat.htm>. | |||
26) Muench, Dawn F., and Michael Rajnik. "Pneumococcal Infections." Emedicine. 16 May 2008. 26 Aug. 2008 <http://www.emedicine.com/med/topic1848.htm>. | |||
27) Nabili, Vishad, Hilary A. Brodie, Nikita I. Neverov, and Steven P. Tinling. "Chronology of Labyrinthitis Ossficans Induced by Streptococcus pneumoniae Meningitis." The Laryngoscope 109 (1999): 931-35. | |||
28) "Outbreak of Pseudomonas aeruginosa Infections Caused by Commercial Piercing of Upper Ear Cartilage." The Journal of the American Medical Association 291 (2004): 981-985. | |||
29) Ronna Staley, MD, James J. Fitzgibbon, MD, Catherine Anderson, and LSM, MSN. "Auricular Infections Caused by High Ear Piercing in Adolescents." Pediatrics. 99 (1997):610-611. | |||
30) Schunknecht, Harold F. "Pathology of the Ear" Lea and Febiger 1993 | |||
31) Stroman DW, Roland PS, Dohar J, Burt W. "Microbiology of normal external auditory canal." Laryngoscope. 111 (2001):2054-9. | |||
32) Tano K, Grahn-Håkansson E, Holm SE, Hellström S. "Inhibition of OM pathogens by alpha-hemolytic streptococci from healthy children, children with SOM and children with rAOM." Int J Pediatr Otorhinolaryngol. 56(2000):185-90. | |||
33) Todar, Kenneth. "Haemophilus influenza". Todar's Online Textbook of Bacteriology | |||
University of Wisconsin-Madison Department of Bacteriology. 2008<http://www.textbookofbacteriology.net/haemophilus.html>. | |||
34) Todar, Kenneth. "Pseudomonas aeruginosa." Todar's Online Textbook of Bacteriology. 2008. University of Wisconsin-Madison Department of Bacteriology. 24 Aug.2008 <http://www.textbookofbacteriology.net/pseudomonas.html> | |||
35) Yoon YJ, Park JW, Lee EJ. "Presence of hBD-1 and hBD-2 in human cerumen and external auditory canal skin". Acta Otolaryngol. 10 (2008):1-5. | |||
Edited by Henry Chan, Rose Dinh, Ambica Selvaraj, Lubna Hagisufi, Ban Paulos, Naima Hagiismail students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | |||
Latest revision as of 02:58, 20 August 2010
Intro to the Ear
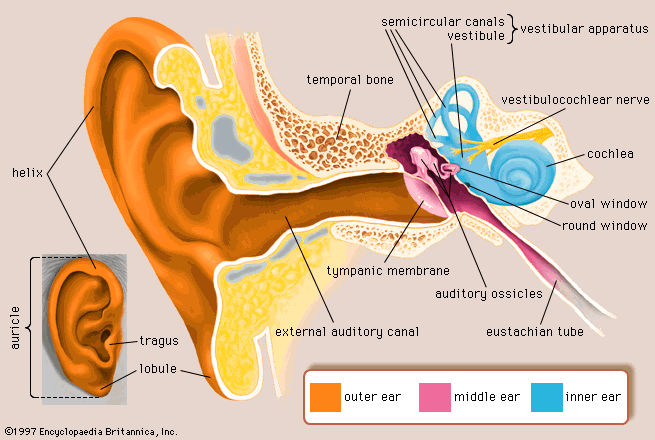
The human ear is comprised of 3 main parts: outer, middle, and inner. The outer ear consists of the pinna(the visible part of the ear) and the auditory ear canal to the tympanic membrane(ear drum). The membrane separates the outer ear from the middle ear which is an air-filled space that is connected to the nasopharynx via the eustachian tube. The middle ear consists of the malleus, incus, and stapes. These three tiny bones carry sound from the ear drum to the inner ear. The inner ear, also known as the bony labyrinth, is a fluid filled compartment that surrounds the membraneous labyrinth which encloses both the cochlea and the vestibular apparatus.(1)
Physical Conditions of the Normal Healthy Ear
The conditions of the pinna are similar to that of the skin. Closer to the ear canal the skin develops a thin lining of ear wax. Ear wax is composed mostly of dead skin cells and keratin with a small mixture of cerumen, sweat, and oil. Cerumen is secreted from the ceruminous glands located in the first third outer part of the ear canal and is thought to be composed mainly of cholesterol, squalene, wax esters, ceramides, and triglycerides. The cerumen also has antimicrobial properties which can be attributed to its slight acidic pH of 5 and the presence of lysozyme. In normal circumstances, the ear wax is continuously pushed out of the ear canal by the slow migration of the top layer of skin cells from the tympanic membrane towards the outer ear. The ear wax traps any foreign particles and organisms on its way out. Ear wax type also varies between people. Generally Native American and Southeast Asians present a dry flaky type of ear wax where as Europeans and Africans produce the "wet type." The dry type falls out easier and is in thinner concentration while the wet type is thicker and stickier, though it should readily fall out as well under normal circumstances. The ear canal is warm(~37C), moist, and abundant with nutrients from dead skin cells. Current research on ear wax shows that some of the antimicrobial properties of the cerumen can be attributed to the presence of antimicrobial peptides, Human Beta-defensin 1 and Human Beta-defensin 2. Other recent studies have shown that cerumen directly inhibits the growth of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans, but its effect on the growth of E. coli remains to be determined (22)(5)(35). In the absence of cerumen, the ear canal would be an optimal environment for microbes.
The middle ear, also known as the tympanic cavity, is an air filled space that is seperated from the outer ear by the tympanic membrane and from the inner ear by the fenestra vestibuli. A mucous membrane lines the middle ear that is continuous with the nasal passageways and nasopharynx via the eustachian tube. This continuity allows the person to equalize the air pressure inside the cavity with that of the outside environment. Mucus has long been known to contain many antimicrobial enzymes and immunoglobulins. In addition to its anti microbial properties, the mucus also serves to trap any foreign particles and microbes that are introduced into the middle ear. Ciliary action of the membrane promptly flushes them out to the nasopharynx. This migration of mucus is important to prevent microbes from colonizing as the tympanic cavity would otherwise present a very favorable environment for microbes. It is moist, abundant in nutrients, protected from the external environment, and at body temperature. (9)(30)
The inner ear consists of two labyrinth structures. The bony labyrinth and the membraneous labyrinth. The bony labyrinth is filled with a fluid called perilymph and encloses the membraneous labyrinth (13). The membraneous labyrinth comprises the vestibular apparatus and the cochlea. It is filled with a seperate fluid that does not mix with the perilymph called endolymph. Both fluids are segregated but derived from blood plasma implying a high concentration of nutrients and a similar pH of ~7.4. In addition to nutrients, small concentrations of immunoglobulins present in blood serum are also found in the perylimph and endolymph, filtered through by the blood-labyrinth barrier (15). A characteristic of perilymph is that its concentration of sodium is high (about 150 milliequivalents per litre) and its concentration of potassium is low (about 5 milliequivalents per litre), similar to other extracellular fluids. Conversely endolymph has a low concentration of sodium (about 15 milliequivalents per litre) and a high concentration of potassium (about 140 milliequivalents per litre) (1). Since the inner ear is completely membrane enclosed, it should be virtually free of bacteria under normal circumstances.
Microbes in the Healthy Ear
Because it is exposed to the outside environment, despite the best efforts of the ceruminous glands, the healthy outer ear still houses a variety of microbes. Some of the most common bacteria are Staphylococcus epidermis, Turicellaotitidis, Alloiococousotitis, Pseudomonas aeruginosa, Corynebacterium, Staphylococcus aureus, and Streptococcus saprophyticum. The most common fungal microbe known to reside in the ear is Candida albicans (29)(7). Under normal circumstances, this bacterial flora does not thrive.
Microbes that are known to inhabit the middle ear are Streptococci, Haemophilus pneumoniae, Moraxella catarrhalis, and less commonly Mycobacterium. These bacteria are known to get to the middle ear by migrating through the nasophraynx and Eustachian tube. Recent research shows that the presence of alpha hemolytic Streptococci from healthy children (as opposed to children with recurrent acute otitis media) inhibit the growth of pathogenic S. pneumoniae and H. influenzae. As a result of this research, there is now an interest in developing a nasal spray that deposits alpha hemolytic Streptococci into the nasopharynx as a means to help prevent rAOM (32).
Because of the blood-labyrinth barrier and the presence of immunoglobulins in the inner ear fluids there should be virtually no microbes living normally in the inner ear.
Normal Changes to the Environment of the Ear
The conditions of the outer ear are subject to the most variation as it is exposed to the outside environment. One of the biggest effects on the environment of the ear would be those that remove the ear wax. This can occur through excessive showering or swimming as water or soap can wash away the thin protective lining of ear wax. Loss of the cerumen encourages the growth of microbes that are normally kept under control by its antimicrobial properties. Use of cotton swaps or hair pins is also common practice as a means to remove ear wax. In addition to the removal of the ear wax, they can also scratch the surface of the skin which can allow microbes to get in to the unprotected tissue. Use of cotton swabs can also push the ear wax further into the canal, which if allowed to build up can become impacted and damage the inner ear canal and the tympanic membrane. These too provide opportunities for microbes to infect the tissue.
Opportunities for microbes to infect the ear diminish as the person ages. Young children with immature immune systems are more susceptible to infection by microbes than are adults with developed immune systems.
Blockage of the Eustachian tube can also increase the likelihood of ear infections. This occurs when the mucus in the tube gets too thick or becomes blocked, as can occur during infections or allergic reactions of the upper respiratory tract. The opening of the Eustachian tube is also affected by head positioning. A head elevation of 20 degrees above horizontal (as can occur while lying down with a pillow) reduces the flow of air to two thirds of normal while a complete horizontal position reduces it to one third. Blockage of the tube prevents a healthy flow of mucus out of the middle ear and as a result, microbes present inside the middle ear become trapped and are allowed to proliferate.(30)
Infections of the Ear
Outer Ear
Auditory Ear Canal
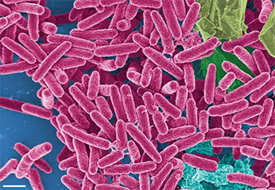
Even though the human ears are exposed to environment conditions they get rarely infected; one can nearly always bathe, swim, and walk in the rain without any problems. This is because the auditory canal is protected by a thin lining of ear wax and the shape of the ear which serves to tip out any fluids (22). Acute external otitis, also known as Swimmer's Ear, is an infection that takes place in the outer auditory canal. Constant exposure to water increases the humidity in the ear. The excess moisture weakens the ear’s external skin and reduces the amount of protective waxes. External otitis can also be caused by swimming in polluted water, scratching the ear or inside the ear, or if an object gets stuck in the ear (2). In these circumstances, bacteria are able to grow and infect the ear canal since they love to live in areas in warm and humid conditions. Even though the outer ear is not as prone to infection as some other parts of the human body it will become infected if, for example, one swims in unsanitized water. Residual dirt and oil from previous swimmers serve as nutrients for organisms like Pseudomonas aeruginosa, the main cause of swimmer's ear, which have simple nutritional requirements. From the water, Pseudomonas aeruginosa is able to swim fast to the ear with its polar flagella. It also uses quorum sensing to detect the density of immediate population which allows them to form biofilms on inanimate surfaces. This bacterium likes to live in aerobic environments; thus, the outer ear is an ideal habitat for this type of organism. This gram negative develops a protective exopolysaccharide coat in biofilm. This gives the bacterium resistance to many antibacterial compounds and antiseptics. It also allows the bacteria to survive harsh conditions including fluctuating temperatures and high salt concentrations (34). The only antibiotics that were found to have some effect on Pseudomonas aeruginosa are gentamicin, imipenem, and fluoroquinolones(34). Less common bacterial causes of swimmer’s ear are Staphylococcus aureus, Proteus mirabilis, Streptococci species, and various gram negative Bacilli. Fungal infections, caused by Aspergillus niger or Candida albicans, are even rarer. According to the current research article, “Staphylococcus aureus Serves as an Iron Source for Pseudomonas aeruginosaa during In Vivo Coculture,” states that pseudomonas aeruginosa uses lysed staphylococcus aureus to get its required amount of iron (21). However, it is still not known if the Pseudonomas aeruginosaa induces the lysing or if Staphylococcus aureus auto lyses.
Ear Piercings
The micro organisms Pseudomonas aeruginosa and Staphylococcus are both involved in the cause of sporadic cartilage infections following ear piercing or other trauma. Pseudomonas aeruginosa is a gram-negative, free-living aerobic bacterium belonging to the bacterial family Pseudomonadaceae. It is commonly found in water and soil and is known to infect human tissues. Pseudomonas aeruginosa is an opportunistic pathogen. This bacterium almost never infects healthy tissues. But if tissue defenses are compromised in some manner, there is hardly any tissue that it cannot infect. Risk for infections increase when newly pierced ears are exposed to Pseudomonas organisms present in lakes or any stagnant water. One way against the activity of these serious infectious organisms is to expose them to the newly discovered fluoroquinoline antibiotic, ciprofloxacin. Ciprofloxacin offers the most antipseudomonal activity against Pseudomonas aeruginosa in vitro. This class of antimicrobial agent also has good activity against staphylococcal species, but resistance can be developed in patients with repeated or prolonged use (28)(29).
Middle Ear
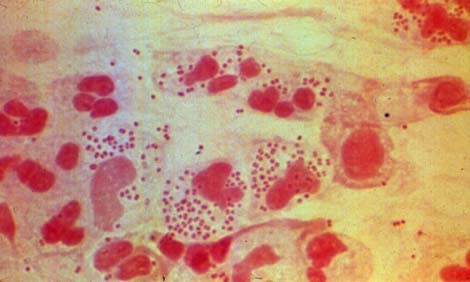
Otitis media is an infection of the middle ear, caused mainly by Haemophilus influenza, Streptocococcus pneumonia, or Moraxella catarrhalis, that mostly infects children at a young age. It is harder for young children to fight the microbe or a bacterium because their immune system is not fully developed. Ear infections are often associated with dysfunction or swelling of the Eustachian tubes. The Eustachian tube is the principal portal for the entry and exit of bacterial in the middle ear. During a viral upper respiratory tract infection(i.e. the common cold), inflammation can block the Eustachian tube. As a result the middle ear does not drain properly and the fluid builds up, becoming a breeding ground for bacteria. Additionally, children have larger adenoid glands than adults. These glands are located behind the throat by the Eustachian tubes and are involved in lymphocyte production. During infections they swell and can block the Eustachian tubes. Furthermore, the adenoid glands themselves can become infected and this infection can directly spread to the tubes (30)(11).
The most common bacteria that grows in the middle ear and causes Otitis media is Haemophilus Influenzae (11). The Haemophilus bacterium grows optimatlly at 35-37C(human body temp.), at a pH of 7.6, and prefers nutrients that can be found in the blood, such as "hemin, NAD or NADP" (33). Based on a recent research article, "Histidine auxotrophy in commensal and disease-causing nontypeable Haemophilus influenza," the lack of histidine is a limiting factor in the growth of bacteria in the middle ear. Because histidine is found in low concentrations in the middle ear, strains of Haimophilus that are predominantly associated with otitis media are highly conserved for an operon encoding histidine producing enzymes (16). Recent studies of Haemophilus influenzae have shown that it can take advantage of the host's immune systems to promote its survival. In a petri dish, it has been shown that Streptococcus pneumoniae out competes Haemophilus by producing peroxides that interfere with its membrane molecules. However, within the respiratory tract of the host, Haemophilus is able to induce an immune response that causes the host to respond only to Streptococcus, thus elminating the competition (18). Moraxella catarrhalis is a gram-negative and oxidase-positive diplococcus which lives in an aerobic environment and is found only in humans. Moraxella catarrhalis is a mucosal pathogen which commonly causes otitis media in young children (8). Its strains can tolerate lower temperature and can grow well at 28ºC(25). Iron plays an important role in helping most microbes survive. However, most of the iron in the human body is coupled to carrier proteins such as transferrin (serum) and lactoferrin (mucosal surface). In order for this bacterium to grow under these limited-iron conditions, Moraxella catarrhalis has to compete for iron by directly utilizing human carrier proteins without any siderophore production (6). This mechanism is similar to H. influenzae and Neisseria species which allow these mucousal pathogens to maintain their growth. With this advantageous feature of being able to utilize these serum carrier proteins, iron-limited M. Catarrhalis can colonize and survive on human mucosal surfaces, as in the middle ear. Prior to otitis media, Moraxella catarrhalis attaches to the respiratory via Type IV pili (TFP). TFP also plays an important part in biofilm formation. From there it migrates to the middle ear via the Eustachian tube to initiate infection (20)(24). Streptococcus pneumoniae is another bacterium that can cause otitis media. Currently there is a study to prevent the child ear infection through the use of a nasal spray. A study on mice Miranda Hitti's lab indicates that the protein lysin can be used to attack Streptococcus pneumonia. Mice infected with Streptococcus pneumoniae and administered the lysin nasal spray did not develop infections where as 80% of the mice that received a placebo did (12).
Inner Ear
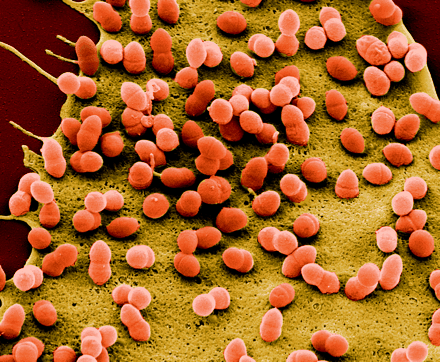
Inner ear infections can be caused by either bacterial or viral infections (14). Since the inner ear is enclosed and is in the deepest part of the ear, it is hard for bacteria to directly affect this area. Bacterial infections of the inner ear arise as a secondary effect of otitis media or bacterial meningitis (an inflammation in the lining of the brain) (14). Sensorineural hearing loss (SNHL) is a critical inner ear complications due to bacterial meningitis. Hearing loss can either occur by direct bacterial invasion or through the passage of bacterial toxins and inflammatory mediators into the inner ear. Streptococcus pneumoniae is one of the most frequently found bacteria to be associated with hearing loss (23)(3).
S. pneumoniae is an extracellular bacterial pathogen enclosed by a capsule. It requires adherence to host cells to gain access to areas where it can initiate infection (26). Normally S. pneunomiae resides in certain areas of the human body without being infectious. However, colonization of this bacterium can become infectious if the organism reaches the auditory tube. S. pneumoniae travels through the blood stream reaching the meninges located around the brain, where it can cause meningitis. They then travel from the cerebrospinal fluid and make their way into membranous labyrinth through the internal auditory canal or the cochlear aqueduct. The membranous labyrinth consists of the vestibule, the semicircular canals, and the cochlea. S. pneumoniae enters the inner ear and initiates ossification, filling the lumen of the cochlea and semicircular canals (27)(10).
Knowledge of the relationship between hearing loss and bacterial meningitis is primarily based on clinical studies. However, many issues revolving this relationship, such as how the bacteria enter the inner ear and their time course for causing hearing loss are still unknown. Length of how long it takes for S. pneumoniae to infect the labyrinth is still uncertain. However, studies done on mice show progressive loss of hearing was identified after 48 hours of exposure to Streptococcus pneumoniae (19)(4).
Viruses have also been known to cause most inner ear attacks, but are usually able to resolve on their own. Vestibular neuritis, the technical term for dizziness, is an inner ear disease caused by a virus. The cause for many inner ear conditions are still unknown. However, current research have shown that certain viruses maybe responsible for infections of specific structures of the inner ear. Herpes simplex virus has been shown to have a link to sudden sensorineural hearing loss. In experiments done these viruses have infected primarily the sensory cells of the labyrinth. Mumps virus have infected principally endolymphatic structures. Influenza virus have shown to infect mesenchymal cells of the perilymphatic channels of the cochlea. These experiments show the possible causality of infections in the inner ear but studies are still being done (19).
References
1) “Anatomy of the Human Ear." Encyclopedia Britannica. 25 Aug. 2008<http://www.britannica.com/EBchecked/topic/175622/ear/65043/Endolymph-and-perilymph#tab=active~checked%2Citems~checked&title=human%20ear%20%3A%3A%20Endolymph%20and%20perilymph%20--%20Britannica%20Online%20Encyclopedia>
2) Badshah, Cyrus. "Medical Encyclopedia." Medline Plus. 22 June 2007. 24 Aug. 2008 <http://www.nlm.nih.gov/medlineplus/ency/article/000622.htm>
3) Bhatt, Samir, Chris Halpin, Wen Hsu, Britt A. Thedinger, Robert A. Levine, Elaine Tuomanen, and Joseph B. Nadol. "Hearing Loss and Pneumococcal Meningitis: An Animal Model." Laryngoscope 101 (1991): 1285-292.
4) Boston, Mark E., and Barry Strasnick. "Inner Ear, Labyrinthitis." Emedicine. 6 Mar. 2008. 25 Aug. 2008 <http://www.emedicine.com/ent/topic226.htm>.
5) Bortz JT, Wertz PW, Downing DT. "Composition of cerumen lipids." J Am Acad Dermatol. 1990 Nov;23(5 Pt 1):845-9.
6) Campagnari, A A, K L Shanks, and D W Dyer. "Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production." Infection and Immunity 62 (1994): 4909-914.
7) Campos A, Arias A, Betancor L, Rodríguez C, Hernández AM, López Aguado D, Sierra A. "Study of common aerobic flora of human cerumen." J Laryngol Otol. 112 (1998 ):613-6
8) Constantinescu, Michael. "Excerpt from Moraxella Catarrhalis Infections." Emedicine. 2 July 2008. 26 Aug. 2008 <http://www.emedicine.com/med/topic1500.htm>.
9) Crouch, James. "Functional Human Anatomy" Lea and Febiger 1978.
10) Dichgans, M., L. Jager, T. Mayer, K. Schorn, and H. Pfister. "Bacterial Meningitis in Adults." Neurology. 28 Nov. 1998. University of California San Diego. 24 Aug. 2008 <http://www.neurology.org/cgi/content/full/52/5/1003>.
11) Haemophilus influenzae type b. The Pink Book: Revisions to 10th Edition, Epidemiology and Prevention of Vaccine Preventable Diseases. March 2008. <http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hib.pdf>.
12) Hitti, Miranda. "Nasal Spray May Prevent Ear Infection." WebMD. 23 Mar. 2007. 27 Aug. 2008 <http://www.webmd.com/cold-and-flu/ear-infection/news/20070323/nasal-spray-may-prevent-ear-infection>.
13) "Inner Ear Fluid Imbalance." Loyola Medicine. 26 Aug. 2008 <http://www.meddean.luc.edu/depts/otolaryn/patient_ed/pdf/ent%20inner%20ear%20fluid%20imbalance.pdf>.
14) "Inner-ear infection-Labyrinthitis." Formula Medical Group. 24 Aug. 2008 <http://www.formulamedical.com/topics/head&neck/inner%20ear%20infection%20labyrinthitis.htm>.
15) Joel M. Bernstein, Pearay L. Ogra. "Immunology of the Ear" Raven Press New York 1987
16) Juliao PC, Marrs CF, Xie J, Gilsdorf JR. "Histidine auxotrophy in commensal and disease-causing nontypeable Haemophilus influenza." Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA. 11 May 2007. 28 Aug. 2008 <http://www.ncbi.nlm.nih.gov/pubmed/17496076?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum>.
18) Lysenko E, Ratner A, Nelson A, Weiser J (2005). "The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces". PLoS Pathog 1 (1): e1. doi:10.1371/journal.ppat.0010001
19) Le, Davis, and Johnson RT. "Experimental viral infections of the inner ear.I.Acute infections of the newborn hamster labyrinth." Lab Investigation 34 (1976): 349-56.
20) Luke, Nicole R., Joseph A. Jurcisek, and Lauren O. Bakaletz. "Contribution of Moraxella catarrhalis Type IV Pili to Nasopharyngeal Colonization and Biofilm Formation." Infection and Immunity 17 (2007): 5559-564.
21) Mashburn, Lauren M., Amy M. Jett, Darrin R. Akins, and Marvin Whiteley."Staphylococcus aureus Serves as an Iron Source for Pseudomonas aeruginosa during In Vivo Coculture." Journal of bacteriology 187 (1994): 554-66.
22) Mayo Clinic Staff. "Swimmer's ear." Mayo clinic.com. 16 Oct. 2006. 24 Aug. 2008<http://www.mayoclinic.com/health/swimmers-ear/DS00473>
23) Miller, Martha L., Aditya H. Gaur, and Ashir Kumar. "Meningitis, Bacterial." Emedicine. 4 Jan. 2008. 25 Aug. 2008 <http://www.emedicine.com/ped/topic198.htm>.
24) "Moraxella Catarrhalis Antigen, Corresponding Gene and Uses Thereof." WIPO. 23 Aug. 2008 <http://www.wipo.int/pctdb/en/wo.jsp?ia=wo2001019996&display=desc>.
25) "Moraxella catarrhalis." Centers for Disease Control and Prevention. 27 Aug. 2008 <http://www.cdc.gov/std/gonorrhea/lab/mcat.htm>.
26) Muench, Dawn F., and Michael Rajnik. "Pneumococcal Infections." Emedicine. 16 May 2008. 26 Aug. 2008 <http://www.emedicine.com/med/topic1848.htm>.
27) Nabili, Vishad, Hilary A. Brodie, Nikita I. Neverov, and Steven P. Tinling. "Chronology of Labyrinthitis Ossficans Induced by Streptococcus pneumoniae Meningitis." The Laryngoscope 109 (1999): 931-35.
28) "Outbreak of Pseudomonas aeruginosa Infections Caused by Commercial Piercing of Upper Ear Cartilage." The Journal of the American Medical Association 291 (2004): 981-985.
29) Ronna Staley, MD, James J. Fitzgibbon, MD, Catherine Anderson, and LSM, MSN. "Auricular Infections Caused by High Ear Piercing in Adolescents." Pediatrics. 99 (1997):610-611.
30) Schunknecht, Harold F. "Pathology of the Ear" Lea and Febiger 1993
31) Stroman DW, Roland PS, Dohar J, Burt W. "Microbiology of normal external auditory canal." Laryngoscope. 111 (2001):2054-9.
32) Tano K, Grahn-Håkansson E, Holm SE, Hellström S. "Inhibition of OM pathogens by alpha-hemolytic streptococci from healthy children, children with SOM and children with rAOM." Int J Pediatr Otorhinolaryngol. 56(2000):185-90.
33) Todar, Kenneth. "Haemophilus influenza". Todar's Online Textbook of Bacteriology University of Wisconsin-Madison Department of Bacteriology. 2008<http://www.textbookofbacteriology.net/haemophilus.html>.
34) Todar, Kenneth. "Pseudomonas aeruginosa." Todar's Online Textbook of Bacteriology. 2008. University of Wisconsin-Madison Department of Bacteriology. 24 Aug.2008 <http://www.textbookofbacteriology.net/pseudomonas.html>
35) Yoon YJ, Park JW, Lee EJ. "Presence of hBD-1 and hBD-2 in human cerumen and external auditory canal skin". Acta Otolaryngol. 10 (2008):1-5.
Edited by Henry Chan, Rose Dinh, Ambica Selvaraj, Lubna Hagisufi, Ban Paulos, Naima Hagiismail students of Rachel Larsen