Ebola Virus NEU2011: Difference between revisions
No edit summary |
No edit summary |
||
| (35 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
<h1><b>Ebola Virus</b></h1> | {{Uncurated}} | ||
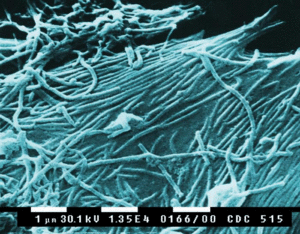
<h1><b>Ebola Virus</b></h1><p align="top">[[File:Ebola_img_2.gif|300px|thumb|right|a. Scanning electron micrograph (SEM) depicts a number of Ebola virions (1/3/2008)]] | |||
< | <h3>Classification</h3> | ||
[[ | ---- | ||
[[ | <font size="2"><b>Class:</b> Class V - ssRNA negative-strand viruses<br> | ||
[[ | <b>Order:</b> Mononegavirales<br> | ||
<b>Family</b>: Filoviridae<br> | |||
<b>Genus:</b> <i>Ebolavirus</i><br> | |||
<b>Species:</b> Zaire ebolavirus</font> | |||
[1](based on Baltimore classification system)<br> | |||
<div id="Description_and_Significance"><h3>Description and Significance</h3></div>[[File:Ebola_img_3.jpg|200px|thumb|right|b. <i>Ebolavirus</i> (2/11/2009)]] | |||
---- | |||
The viral family Filoviridae consists of two distinct genuses, <i>Marburgvirus</i> and <i>Ebolavirus</i>. The family name, from the Latin "filum" meaning "thread" or "filament", describes the threadlike structure of the organisms. Filoviruses are negative-strand RNA viruses, usually between 850 and 920nm in length [2]. In the <i>Ebolavirus genus</i>, four distinct species have been identified, three of which co-circulate in Africa, and are known human pathogens [3]. </p> | |||
<p>The Ebola virus (EBOV) strains were first identified in Africa in 1976. Two epidemics occurred almost simultaneously in Zaire and Sudan, killing almost 500 people. In Zaire, an outbreak at a local hospital, possibly due to contaminated hypodermic needles, exhibited an 88% mortality rate for those infected. The predominant symptoms of the virus were hemorrhagic fever and damage to the endothelial cells that form the linings of blood vessels, characterized by severe internal and sometimes external bleeding. The responsible strain was identified and named <i>Ebola</i>, after the Ebola River region where the epidemic occurred. This strain of the virus is referred to as the Zaire virus, and is the type species for the genus [4,5]. </p> | |||
<p>Since its discovery over 30 years ago, EBOV research has elucidated much about the mechanism by which an Ebola outbreak occurs. Since the epidemic in Zaire, several more cases have been observed in humans and animals, though no other strain has proved as deadly as that isolated from the Ebola River area [3]. While research has significantly expanded knowledge about how the virus survives between epidemics and how it is passed to human populations, much is still unknown about EBOV. With the modern advent of biological warfare, the contagiousness and lethality of EBOV makes it a potential weapon of bioterrorism [6]. Therefore, further research is important to understand and hopefully identify a vaccine for EBOV, to protect against future epidemics.</p> | |||
<div id="Genome_Structure"><h3>Genome Structure</h3></div><p align="bottom">[[File:Ebola img 6.jpg|300px|thumb|right|c. Structure of <i>Ebolavirus</i> genome (2/11/2009)]] | |||
---- | |||
The Ebola genome, as with that of other members of Mononegavirales, is a non-segmented and consists of a single molecule of linear, negative-strand ssRNA. The genome is approximately 19kb long, and constitutes about 1.1% of the viral weight [1, 7, 8].</p> | |||
<p>The Ebola genome has 7 known nucleotide sequences that code for structural and non-structural proteins also known as VPs. The core of the virus is made up of RNA genomic molecules comprised of nucleoprotein (NP) [7].</p> | |||
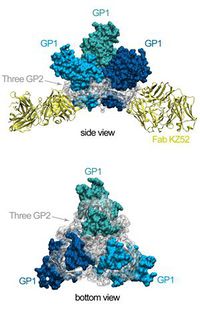
<p>There are several types of VPs, each with a different function. VP30 proteins play an important role in RNA transcription activation, which is strongly dependent on the concentration of VP30. VP24 the primary matrix protein, and is the most abundant virion component. Its role is unclear. VP35 plays an important role in viral RNA synthesis. It acts as a type of interferon antagonist. VP40 is a matrix protein from the negative strand of RNA. It mainly participates in the assembly of lipid-enveloped viruses by providing a link between the surrounding membrane and the nucleocapsid structure. The protein GP, also known as single surface transmembrane GP, is responsible for attachment and entry into the target cell. The L protein is a non-segmented negative strand RNA, it plays an essential role in catalyzing transcription. The L protein is a RNA-dependent RNA polymerase [1, 7]. [[File:GP_protein_structure.jpg|200px|thumb|right|d. Structure of EBOV glycoprotein (GP) [9]]] </p> | |||
<p> All gene expression required for viral replication is carried out within the Ebola virion, with no participation by the host-cell enzymes. Nucleotide sequences at the 3’-terminus are complimentary to similar regions of genetic code in the 5’-end, which does not have a cap. The 3’-end also has no poly(A) trail. Transcription occurs from the 3’-end and results in seven to nine mRNA strands. The Ebola amino acids identity is distinct from that of other Filoviridae viruses, evidenced by its unique method of entry into the host-cell [1, 7].</p> | |||
<h3> Structure and Metabolism </h3> | |||
---- | |||
<p> The EBOV is an enveloped, non-segmented, negative-strand RNA virus. Glycoprotein is the sole resident of the EBOV surface, and is made up of carbohydrate chains called glycans. In its active form, EBOV glycoprotein (GP) has 2 subunits with separate structural and functional roles: GP1, which effects the attachment to host cells, and GP2, which mediates fusion of viral and host membranes. EBOV is thought to enter host cells by receptor-mediated endocytosis through clathrin-coated pits and caveolae, followed by actin- and microtubule-dependent transport to the endosome [9].</p> | |||
<p>Crystal structure reveals that most of GP is shielded by a thick coat of carbohydrates and identified very few sites left exposed and available for antibody binding [9]. </p> | |||
<h3> Ecology </h3> | |||
---- | |||
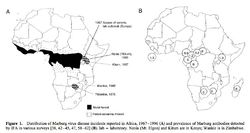
[[File:ebola_ecology.jpg|250px|thumb|right|e. Distribution of Marbug virus diseas incidents in Africa 1967-1996 [10]]] | |||
<p> EBOV thrives in tropical regions where rivers and lakes are surrounded by dense vegetation. Based on outbreak frequency, the environment and climate in sub-Saharan African appears most ideal – the vast majority of EBOV outbreaks occurred in this region. Also, the lack of effective sanitation and poor living conditions of this region contribute to the spread of the virus [10].</p> | |||
<p> [[File:ebola_reservoir_table.jpg|250px|thumb|right|f. Instances of Marburg virus transmission via bats in Africa, 1980 and 1987 [10]]] Possible reservoirs for EBOV include bats, monkeys, and spiders. This conclusion was made based on multiple cases where cultures from bites by these few animals contained EBOV. Bats as a reservoir presents an unusual opportunity for the virus to transfer itself to arthropods as well as a multitude of mammals who could have contact with humans [10].</p> | |||
< | <h3> Pathology </h3> | ||
< | ---- | ||
< | <p>EBOV is an extremely aggressive pathogen, causing hemorrhagic fever syndrome in humans and non-human primates. Since its recognition in 1976, semi-regular outbreaks in Africa have exhibited 50-90% mortality rates. There is no treatment or vaccine for EBOV, and due to the sampling difficulty and highly hazardous nature of the virus, little is known about its specific mechanism of pathogenicity. However, the symptoms and clinical course of infection are well documented [11].</p> | ||
< | <p> Research indicates that the viral GP is an important factor in EBOV infection. The two forms of GP synthesized by the virus have different structural and chemical properties, and both appear to contribute to infection in different ways. The full-length transmembrane GP binds preferentially to endothelial cells in the lining of blood vessels, causing sever cell damage and the mass hemorrhagic associated with the disease. Secreted GP (sGP) appears to interact with the host cell neutrophils, which may interfere with the immune system’s ability to recognize the virus in the early days of infection. This evasion, coupled with EBOV’s unusually high replication rate, quickly overwhelms the host’s immune system [11]. </p> | ||
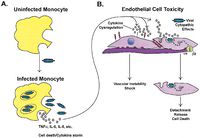
<p> The EBOV infection generally lasts 2 to 3 weeks. Early symptoms mimic the flu and include fever and malaise. However, rapid disease progression leads to severe bleeding, reduced blood coagulation, and a diverse array of other hematological symptoms such as lymphopenia. Late in the course of the disease, the virus attacks the liver and vascular endothelium, leading to the diffuse bleeding and hypotensive shock that most often are the direct cause of death [11]. [[File:infection.jpg|200px|thumb|right|g. Infection of host endothelial cell by EBOV [11]]]</p> | |||
<h3> Current Research </h3> | |||
---- | |||
<p> In recent years, EBOV research has received an increase attention due to its re-emergence in central Africa. EBOV is one of the most lethal human pathogens in the world. Due to its lethality, it is afraid that it would be used as a biological weapon.</p> | |||
<p> Currently, EBOV research focuses on the virus’ natural reservoir as well as its methods of transference. Due to the various challenges in studying such a lethal pathogen, little is known about the virus’ natural habitat or how it is spread. Research developing anti-viral medicines and vaccines against EBOV are also underway.</p> | |||
<p> Research on an EBOV vaccine has been underway for quite some time. In a recent break-through, researchers at the Howard Hughes Medical Institute infected guinea pigs with the EBOV and injected them with a DNA vaccine made up of plasmids containing the EBOV protein. The animals resisted infection for two to four months after the immunization with the experimental vaccine [12].The virus vaccine itself creates an immune response to the proteins of the outer envelope. This is initiated by injecting the vaccine into the muscle tissue of the animal and allowing the plasmid for this protein to be taken up by the animal cell. Once expressed, the immune system’s T cell based response was sufficient enough to stop the virus [12].</p> | |||
<p> Another area of interest is focused on post-exposure due to its possible use as a biological weapon. Recently, scientists have successfully tested the efficacy of vesicular stomatitis virus-based EBOV vaccine vector as a post-exposure treatment in three different animal models (Guinea pig, mouse, and rhesus macaques). The results of this research shows that in the guinea pig and mouse models, it was possible to protect 50% and 100% of the animals if the animals were treated as late as 24 hours after EBOV exposure. On the other hand, 4 out of 8 rhesus macaques were protected if treated 20 to 30 min following exposure. Vesicular stomatitis virus-based EBOV vaccine vector can be a very effective post-exposure treatment strategy for EBOV, continuous research may lead to a vaccine that may be effective for humans [13].</p> | |||
<h3> Fun Fact </h3> | |||
---- | |||
<p> In 1995, the US Army Medical Research Institute of Infectious Disease filed a report entitled "Lethal Experimental Infections of Rhesus Monkeys by Aerosolized Ebola Virus". Using a head-only exposure aerosol system, droplets containing the virus were administered to the respiratory system of anesthetized monkeys. Three different experimental treatments were applied: low dose exposure, high dose exposure, and exposure to a cell culture infected with the virus [14].</p> | |||
<p> All monkeys in the experimental groups came down with the disease, confirmed to be the same syndrome observed in monkeys infected with non-aerosolized EBOV. However, even in the low exposure group, the symptoms of the resulting hemorrhagic fever were reminiscent of the most severe cases documented in humans, and also included previously unobserved symptoms such as subcutaneous and venipuncture site bleeding and serosanguinous nasal discharge . Disease caused by inhalation of the virus also causes a more rapidly lethal disease, killing subjects in the low exposure group within 4 to 5 days from onset of clinical symptoms (about 9 days post-exposure)[14]. | |||
<div id="References"><h3>References</h3></div> | |||
---- | |||
# ICTVdB Management (2006). 01.025.0.02.[http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/ Ebolavirus]. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA <br> | |||
# Regnery, R.L., K.M. Johnson, and M.P. Kiley, Marburg and Ebola viruses: possible members of a new group of negative-strand viruses. The replication of negative-strand viruses, 1981: p. 971-977.<br> | |||
# Pourrut, X., et al., [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VPN-4G5YSRB-3&_user=10&_coverDate=06/30/2005&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=f98ee2d85441514b0b9be0fb815ab365&searchtype=a/ The natural history of Ebola virus in Africa]. Microbes and Infection, 2005. 7: p. 1005-1014. | |||
# McCormick, J.B., et al., [http://www.jstor.org/pss/30115071/ Biologic Differences between Strains of Ebola Virus from Zaire and Sudan]. The Journal of Infectious Diseases, 1983. 147(2): p. 264-267.<br> | |||
# Heymann, D.L., et al., [http://www.jstor.org/pss/30115146 Ebola Hemorrhagic Fever: Tandala, Zaire, 1977-1978]. The Journal of Infectious Diseases, 1980. 142(3): p. 372-376.<br> | |||
# Groseth, A., et al., [http://www.springerlink.com/content/mg1677m19137272u/ Hemorrhagic Fever Viruses as Biological Weapons], in Bioterrorism and Infectious Agents: A New Dilemma for the 21st Century, I.W. Fong and K. Alibek, Editors. 2009, Springer New York. p. 169-191.<br> | |||
# Ascenz, P., et al. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T9P-4PYJSCP-1&_user=2403224&_coverDate=06/30/2008&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1651700848&_rerunOrigin=google&_acct=C000057194&_version=1&_urlVersion=0&_userid=2403224&md5=573ea102582eebcb0286dc3719b41f1b&searchtype=a/ Ebolavirus and Marburgvirus: Insight the Filoviridae Family]. Molecular Aspects of Medicine. 2008. 29(3): p. 151-85.<br> | |||
# Paustian. "19-20 Ebola Is a Filamentous Virus with a Single-stranded RNA Genome." The Microbial World :: A Look at All Things Small. 08 Nov. 2008. Web. 21 Feb. 2011. [http://www.microbiologytext.com/index.php?module=Book&func=displayarticle&art_id=494/ link]<br> | |||
# Lee, J.E. et al. [http://www.nature.com/nature/journal/v454/n7201/abs/nature07082.html Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor]. Nature, 2008. 454(7201): 177-182 | |||
# Monath, T.P. [http://www.ncbi.nlm.nih.gov/pubmed/9988176 Ecology of Marburg and Ebola Viruses: speculations and directions for future research]. 1999. 179(1): 127-138. | |||
# Sullivan, N., Zhi-Young Y. and G. J. Nabel. [http://jvi.asm.org/cgi/reprint/77/18/9733.pdf Ebola Virus Pathogenesis: Implications for Vaccines and Therapies]. Journal of Virology. 2003. 77(18): p.9733-9737. | |||
# Ling, X. et al. [http://www.nature.com/nm/journal/v4/n1/pdf/nm0198-037.pdf Immunization for Ebola Virus Infection]. Nature. 1998. 4(1): 37-42. | |||
# Feldmann, H. et al. [http://www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.0030002 Effective Post-Exposure treatments of Ebola infection. PLos Pathogen]. 2007. 3(1): 54-61. | |||
# US Army Medical Research Institute of Infectious Disease. [http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA292410&Location=U2&doc=GetTRDoc.pdf Lethal Experimental Infections of Rhesus Monkeys by Aerosolized Ebola Virus]. US Army Medical Research and Development Command, Frederick, MD. | |||
Images <b>a, b</b> courtesy of [http://bepast.org/dataman.pl?c=lib&dir=docs/photos/ Center for Biological Counterterrorism and Emerging Diseases CBC-ED image gallery]<br>Image <b>c</b> courtesy of [http://www.microbiologytext.com/index.php?module=Book&func=displayarticle&art_id=494/ The Microbial World] | |||
Latest revision as of 18:21, 15 April 2011
Ebola Virus
Classification
Class: Class V - ssRNA negative-strand viruses
Order: Mononegavirales
Family: Filoviridae
Genus: Ebolavirus
Species: Zaire ebolavirus
[1](based on Baltimore classification system)
Description and Significance
The viral family Filoviridae consists of two distinct genuses, Marburgvirus and Ebolavirus. The family name, from the Latin "filum" meaning "thread" or "filament", describes the threadlike structure of the organisms. Filoviruses are negative-strand RNA viruses, usually between 850 and 920nm in length [2]. In the Ebolavirus genus, four distinct species have been identified, three of which co-circulate in Africa, and are known human pathogens [3].
The Ebola virus (EBOV) strains were first identified in Africa in 1976. Two epidemics occurred almost simultaneously in Zaire and Sudan, killing almost 500 people. In Zaire, an outbreak at a local hospital, possibly due to contaminated hypodermic needles, exhibited an 88% mortality rate for those infected. The predominant symptoms of the virus were hemorrhagic fever and damage to the endothelial cells that form the linings of blood vessels, characterized by severe internal and sometimes external bleeding. The responsible strain was identified and named Ebola, after the Ebola River region where the epidemic occurred. This strain of the virus is referred to as the Zaire virus, and is the type species for the genus [4,5].
Since its discovery over 30 years ago, EBOV research has elucidated much about the mechanism by which an Ebola outbreak occurs. Since the epidemic in Zaire, several more cases have been observed in humans and animals, though no other strain has proved as deadly as that isolated from the Ebola River area [3]. While research has significantly expanded knowledge about how the virus survives between epidemics and how it is passed to human populations, much is still unknown about EBOV. With the modern advent of biological warfare, the contagiousness and lethality of EBOV makes it a potential weapon of bioterrorism [6]. Therefore, further research is important to understand and hopefully identify a vaccine for EBOV, to protect against future epidemics.
Genome Structure
The Ebola genome, as with that of other members of Mononegavirales, is a non-segmented and consists of a single molecule of linear, negative-strand ssRNA. The genome is approximately 19kb long, and constitutes about 1.1% of the viral weight [1, 7, 8].
The Ebola genome has 7 known nucleotide sequences that code for structural and non-structural proteins also known as VPs. The core of the virus is made up of RNA genomic molecules comprised of nucleoprotein (NP) [7].
There are several types of VPs, each with a different function. VP30 proteins play an important role in RNA transcription activation, which is strongly dependent on the concentration of VP30. VP24 the primary matrix protein, and is the most abundant virion component. Its role is unclear. VP35 plays an important role in viral RNA synthesis. It acts as a type of interferon antagonist. VP40 is a matrix protein from the negative strand of RNA. It mainly participates in the assembly of lipid-enveloped viruses by providing a link between the surrounding membrane and the nucleocapsid structure. The protein GP, also known as single surface transmembrane GP, is responsible for attachment and entry into the target cell. The L protein is a non-segmented negative strand RNA, it plays an essential role in catalyzing transcription. The L protein is a RNA-dependent RNA polymerase [1, 7].
All gene expression required for viral replication is carried out within the Ebola virion, with no participation by the host-cell enzymes. Nucleotide sequences at the 3’-terminus are complimentary to similar regions of genetic code in the 5’-end, which does not have a cap. The 3’-end also has no poly(A) trail. Transcription occurs from the 3’-end and results in seven to nine mRNA strands. The Ebola amino acids identity is distinct from that of other Filoviridae viruses, evidenced by its unique method of entry into the host-cell [1, 7].
Structure and Metabolism
The EBOV is an enveloped, non-segmented, negative-strand RNA virus. Glycoprotein is the sole resident of the EBOV surface, and is made up of carbohydrate chains called glycans. In its active form, EBOV glycoprotein (GP) has 2 subunits with separate structural and functional roles: GP1, which effects the attachment to host cells, and GP2, which mediates fusion of viral and host membranes. EBOV is thought to enter host cells by receptor-mediated endocytosis through clathrin-coated pits and caveolae, followed by actin- and microtubule-dependent transport to the endosome [9].
Crystal structure reveals that most of GP is shielded by a thick coat of carbohydrates and identified very few sites left exposed and available for antibody binding [9].
Ecology
EBOV thrives in tropical regions where rivers and lakes are surrounded by dense vegetation. Based on outbreak frequency, the environment and climate in sub-Saharan African appears most ideal – the vast majority of EBOV outbreaks occurred in this region. Also, the lack of effective sanitation and poor living conditions of this region contribute to the spread of the virus [10].
Possible reservoirs for EBOV include bats, monkeys, and spiders. This conclusion was made based on multiple cases where cultures from bites by these few animals contained EBOV. Bats as a reservoir presents an unusual opportunity for the virus to transfer itself to arthropods as well as a multitude of mammals who could have contact with humans [10].
Pathology
EBOV is an extremely aggressive pathogen, causing hemorrhagic fever syndrome in humans and non-human primates. Since its recognition in 1976, semi-regular outbreaks in Africa have exhibited 50-90% mortality rates. There is no treatment or vaccine for EBOV, and due to the sampling difficulty and highly hazardous nature of the virus, little is known about its specific mechanism of pathogenicity. However, the symptoms and clinical course of infection are well documented [11].
Research indicates that the viral GP is an important factor in EBOV infection. The two forms of GP synthesized by the virus have different structural and chemical properties, and both appear to contribute to infection in different ways. The full-length transmembrane GP binds preferentially to endothelial cells in the lining of blood vessels, causing sever cell damage and the mass hemorrhagic associated with the disease. Secreted GP (sGP) appears to interact with the host cell neutrophils, which may interfere with the immune system’s ability to recognize the virus in the early days of infection. This evasion, coupled with EBOV’s unusually high replication rate, quickly overwhelms the host’s immune system [11].
The EBOV infection generally lasts 2 to 3 weeks. Early symptoms mimic the flu and include fever and malaise. However, rapid disease progression leads to severe bleeding, reduced blood coagulation, and a diverse array of other hematological symptoms such as lymphopenia. Late in the course of the disease, the virus attacks the liver and vascular endothelium, leading to the diffuse bleeding and hypotensive shock that most often are the direct cause of death [11].
Current Research
In recent years, EBOV research has received an increase attention due to its re-emergence in central Africa. EBOV is one of the most lethal human pathogens in the world. Due to its lethality, it is afraid that it would be used as a biological weapon.
Currently, EBOV research focuses on the virus’ natural reservoir as well as its methods of transference. Due to the various challenges in studying such a lethal pathogen, little is known about the virus’ natural habitat or how it is spread. Research developing anti-viral medicines and vaccines against EBOV are also underway.
Research on an EBOV vaccine has been underway for quite some time. In a recent break-through, researchers at the Howard Hughes Medical Institute infected guinea pigs with the EBOV and injected them with a DNA vaccine made up of plasmids containing the EBOV protein. The animals resisted infection for two to four months after the immunization with the experimental vaccine [12].The virus vaccine itself creates an immune response to the proteins of the outer envelope. This is initiated by injecting the vaccine into the muscle tissue of the animal and allowing the plasmid for this protein to be taken up by the animal cell. Once expressed, the immune system’s T cell based response was sufficient enough to stop the virus [12].
Another area of interest is focused on post-exposure due to its possible use as a biological weapon. Recently, scientists have successfully tested the efficacy of vesicular stomatitis virus-based EBOV vaccine vector as a post-exposure treatment in three different animal models (Guinea pig, mouse, and rhesus macaques). The results of this research shows that in the guinea pig and mouse models, it was possible to protect 50% and 100% of the animals if the animals were treated as late as 24 hours after EBOV exposure. On the other hand, 4 out of 8 rhesus macaques were protected if treated 20 to 30 min following exposure. Vesicular stomatitis virus-based EBOV vaccine vector can be a very effective post-exposure treatment strategy for EBOV, continuous research may lead to a vaccine that may be effective for humans [13].
Fun Fact
In 1995, the US Army Medical Research Institute of Infectious Disease filed a report entitled "Lethal Experimental Infections of Rhesus Monkeys by Aerosolized Ebola Virus". Using a head-only exposure aerosol system, droplets containing the virus were administered to the respiratory system of anesthetized monkeys. Three different experimental treatments were applied: low dose exposure, high dose exposure, and exposure to a cell culture infected with the virus [14].
All monkeys in the experimental groups came down with the disease, confirmed to be the same syndrome observed in monkeys infected with non-aerosolized EBOV. However, even in the low exposure group, the symptoms of the resulting hemorrhagic fever were reminiscent of the most severe cases documented in humans, and also included previously unobserved symptoms such as subcutaneous and venipuncture site bleeding and serosanguinous nasal discharge . Disease caused by inhalation of the virus also causes a more rapidly lethal disease, killing subjects in the low exposure group within 4 to 5 days from onset of clinical symptoms (about 9 days post-exposure)[14].
References
- ICTVdB Management (2006). 01.025.0.02.Ebolavirus. In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C. (Ed), Columbia University, New York, USA
- Regnery, R.L., K.M. Johnson, and M.P. Kiley, Marburg and Ebola viruses: possible members of a new group of negative-strand viruses. The replication of negative-strand viruses, 1981: p. 971-977.
- Pourrut, X., et al., The natural history of Ebola virus in Africa. Microbes and Infection, 2005. 7: p. 1005-1014.
- McCormick, J.B., et al., Biologic Differences between Strains of Ebola Virus from Zaire and Sudan. The Journal of Infectious Diseases, 1983. 147(2): p. 264-267.
- Heymann, D.L., et al., Ebola Hemorrhagic Fever: Tandala, Zaire, 1977-1978. The Journal of Infectious Diseases, 1980. 142(3): p. 372-376.
- Groseth, A., et al., Hemorrhagic Fever Viruses as Biological Weapons, in Bioterrorism and Infectious Agents: A New Dilemma for the 21st Century, I.W. Fong and K. Alibek, Editors. 2009, Springer New York. p. 169-191.
- Ascenz, P., et al. Ebolavirus and Marburgvirus: Insight the Filoviridae Family. Molecular Aspects of Medicine. 2008. 29(3): p. 151-85.
- Paustian. "19-20 Ebola Is a Filamentous Virus with a Single-stranded RNA Genome." The Microbial World :: A Look at All Things Small. 08 Nov. 2008. Web. 21 Feb. 2011. link
- Lee, J.E. et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature, 2008. 454(7201): 177-182
- Monath, T.P. Ecology of Marburg and Ebola Viruses: speculations and directions for future research. 1999. 179(1): 127-138.
- Sullivan, N., Zhi-Young Y. and G. J. Nabel. Ebola Virus Pathogenesis: Implications for Vaccines and Therapies. Journal of Virology. 2003. 77(18): p.9733-9737.
- Ling, X. et al. Immunization for Ebola Virus Infection. Nature. 1998. 4(1): 37-42.
- Feldmann, H. et al. Effective Post-Exposure treatments of Ebola infection. PLos Pathogen. 2007. 3(1): 54-61.
- US Army Medical Research Institute of Infectious Disease. Lethal Experimental Infections of Rhesus Monkeys by Aerosolized Ebola Virus. US Army Medical Research and Development Command, Frederick, MD.
Images a, b courtesy of Center for Biological Counterterrorism and Emerging Diseases CBC-ED image gallery
Image c courtesy of The Microbial World