Feline panleukopenia virus: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
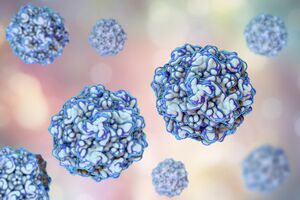
[[Image: | [[Image:Microbeoftheday_FPV.jpg|thumb|300px|right|Feline Panleukopenia Virus. Image credit: Photograph by Kateryna Kon/science Photo Library.]] | ||
Revision as of 17:31, 30 November 2023
Classification
Viruses; Monodnaviria; Shotokuvirae; Cossaviricota; Quintoviricetes; Piccovirales; Parvoviridae; Parvovirinae; Protoparvovirus; Protoparvovirus carnivoran 1
Species
|
NCBI: [1] |
Genus species
Description and Significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
Feline panleukopenia virus (FPV or FPLV) is a non-enveloped, single-stranded DNA virus and is sometimes referred to as feline distemper, cat plague, or feline parvovirus. FPV was first identified in the 1920s and has maintained genomic stability to a degree. FPV is a serious, highly contagious and potentially fatal issue among unvaccinated felines. Following proper vaccine guidelines can prevent felines from getting infected.
Genome Structure
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
The genome of feline panleukopenia virus contains approximately 5123 nucleotides and 2 open-reading frames. The amount of base pairs in the DNA sequence varies depending on the specific strain of the virus. Due to the Y-type structure at the 3-terminal and the U-shaped structure at the 5-terminal, it is challenging for researchers to amplify the full-length genome of this virus and many others in the parvovirus genus.
Cell Structure, Metabolism and Life Cycle
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Parvoviruses, including FPV, do not have DNA polymerases and cannot promote cell division. FPV relies on its host for its metabolic processes and use the host's cell division machinery to complete their lytic cycle.
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
The virus can be transmitted either by the fecal-oral route or by indirect transmission via fomites contaminated by infected individuals, as well as from humans that have the virus on their hands or clothes. The virus can live in an environment for months to a year and in some instances over a year. FPV is also highly resistant to certain disinfectants making it difficult for owners to remove it from their household. Using transferrin receptors FPV can enter cells and replicate inside of cells that are currently in the synthesis phase of mitosis. FPV can occur in cats younger than 1 year old, or unvaccinated or improperly vaccinated cats of any age. Severity of the virus depends on age, immunity of the individual and infections from other bacteria or viruses. FPV causes feline infections enteritis (FIE) which is a severe infection of the gut that is fatal and highly contagious making it spread rapidly in a multiple cat household. In addition to domestic cats, FPV can infect wild cats, raccoons, foxes, and mink. Clinical symptoms of infected individuals are lethargy, diarrhea, inappetence, fever (103°F to 107°F or 39.5°C to 42.5°C), vomiting, weight loss. In severe cases, cats can have ulcers in the mouth as well as exhibit mucosal pallor. In terminal cases, cats can be hypothermic, bradycardic, and comatose. If left untreated or if the virus has progressed too far then death can occur usually because of dehydration, low blood sugar, imbalanced electrolytes, hemorrhaging, or bacteria in the bloodstream.
References
Author
Page authored by Phoebe Chambers, student of Prof. Bradley Tolar at UNC Wilmington.