Ferroplasma acidiphilum: Difference between revisions
No edit summary |
|||
| Line 7: | Line 7: | ||
''Ferroplasma acidiphilum'' | ''Ferroplasma acidiphilum'' | ||
<small>* Multiple strains of this organism have been isolated since its discovery (Y<sup>T</sup>, Y-2 etc). Majority of the information on this page are for the strain YT unless otherwise specifically mentioned.</small> | <small>* Multiple strains of this organism have been isolated since its discovery (Y<sup>T</sup>, Y-2 etc). Majority of the information on this page are for the strain YT unless otherwise specifically mentioned.</small> | ||
Revision as of 13:45, 25 April 2010
Classification
Domain (Archaea); Phylum (Euryarchaeota); Class (Thermoplasmata); Order (Thermoplasmatales); Family (Ferroplasmaceae); Genus (Ferroplasma)
Species
Ferroplasma acidiphilum
* Multiple strains of this organism have been isolated since its discovery (YT, Y-2 etc). Majority of the information on this page are for the strain YT unless otherwise specifically mentioned.
Description and Significance
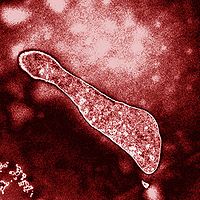
Ferroplasma acidiphilum is a species of an iron-oxidizing, acidophilic, chemolithoautotrophic archaea. It lives in a metal-heavy environment containing high levels of iron and sulfur at a very acidic pH. It has been categorized as an extremophile as it grows optimally at a pH of 1.7. It was first isolated from a bioreactor pilot plant in Tula, Russia [Golyshina et. al. 2000]. The bioreactor was used to leach gold from pyrite-ore, a chemical reaction that F. acidiphilum plays a role in. It is part of the order Thermoplasmata that contains other acidophilic genera including Picrophilus and Thermoplasma. F. acidiphilum and its sister species F. acidarmanus play a large role in geochemical cycling of iron and sulfur in very acidic, metal-heavy habitats both natural and man-made [Golyshina et. al. 2000]. It has been found that some of its intracellular enzymes function optimally at pH levels as low as 1.7, much lower than what the actual cytoplasmic pH level of 5.6 [Pivovarova et. al. 2002]. This has led to research aimed at determining how this "pH anomaly" exists and how this organism has evolved to survive this way. It is also non-motile as it lakes a flagella [Golyshina 2000].
Phylogeny and Genomics
archaea - circular. Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence? Genome not sequenced yet. But, RFLP combined with pulse field gel electrophoresis - result - genome fragment length different from close acidophilic relatives placed under the same order. Sister species genome sequenced - length. 16S rRNA analysis - that places this org in this branch F. acidiphilum has not had its genome sequenced as of yet, but some DNA studies have been performed that have allowed for phylogenetic analysis. Characterization of F. acidiphilum has placed it in a new family (Ferroplasmaceae) and genus (Ferroplasma). The new family was justified because of its >10% difference in 16S rRNA sequence from its closest relatives in the families Thermoplasmaceae and Picrophilaceae of which it only shares 87.2 and 89.9% similarity respectively [Golyshina et. al. 2000]. It was also distinct because unlike other members of the order Thermoplasmales, it lacks any form of a cell wall [Golyshina 2000]. The two strains of the species (Y-T and Y-2) have approximately 35 mol % G+C content in their genome.
Life Cycle, Cell Structure, and Metabolism
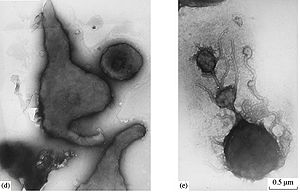
F. acidiphilum cells can come in a variety of forms depending on environmental conditions and growth stage [add ref]. At the beginning of the exponential growth phase cells have irregular round shapes. As growth continues cells can take on other unusual shapes, including a vibrio-like form. Cells in the stationary phase are pleomorphic and assume irregular shapes [Pivovarova et. al. 2002]. Cells also take on irregular shapes when budding. Because of the large number of forms a F. acidiphilum cell can take, cell sizes vary greatly but typically have a diameter of 0.3-3μm (Golyshina and Timmis 2005).
All cells in the Ferroplasma genus lack cell walls [add ref]. Under acidic conditions the ether-linked membrane lipids of archaea are more resistant to acid hydrolysis than the ester-linked membrane lipids of Bacteria and Eukaryotes. F. acidiphilum membranes are mainly composed of unique tetraether lipids that form a monolayer giving the membrane more stability. One of the main constituents of the membrane, β-D-glucopyranosylcaldarchaetidylglycerol, comprises 55% of the membrane and contains cyclopentane rings, giving the membrane more rigidity (batrakov 2002). The membrane composition of F. acidiphilum and other acidophilic archaea cells create large proton gradients due to their low proton permeability, allowing the cells to survive at very acidic pH levels. Membrane proteins of this species function optimally up to 3 pH levels below the mean cytoplasmic pH of 5 (macalady).
F. acidiphilum utilizes iron via assimilatory and dissimilatory mechanisms. Though iron is the 4th abundant element in the Earth's crust and is crucial to living cells, it is often a limiting nutrient being low in solubility and poor bioavailability (Ferrer M et. al. 2007). An exception to this are the iron-rich acidic environments like the ones habited by F. acidiphilum. An exception to this are the iron-rich acidic environments like the ones habited by F. acidiphilum. Interestingly F. acidiphilum has been found to have iron-protein dominated cellular machinery while such similar iron-proteins have not been found in other organisms living in similar environments. 86% of the investigated 189 cellular proteins of F. acidiphillum are found to be iron proteins (Ferrer M et. al. 2007) with the iron acting as 'iron rivets' to stabilize the three dimensional structures of these proteins. These discoveries lead to an intriguing possibility of it being evolved entirely within the iron rich habitat with its metalloproteins being a living representation of early cellular life.
F. acidiphilum YT obtains energy for life by oxidation of ferrous iron availabed as pyrite (FeS2) or Ferrous Sulfate (FeSO4) [Golyshina et. al. 2000]. It uses oxygen as its terminal electron acceptor. It obtains its carbon from carbondioxide (inorganic) and is also capable of utilizing carbon from yeast extracts (organic) under laboratory conditions (Dopson M et. al. 2004). Thus it could be appropriately termed as a chemolithoautotroph with mixotrophic abilities. It grow at a temperature range of 15-45 ˚C and pH range of 1.3 to 2.2. The optimal temperature for growth is 35-36˚C and the optimal pH is 1.7. With its ability to oxidize iron, F. acidiphilum plays a role in the global geochemical iron cycle (Figure 4).
Ecology and Pathogenesis
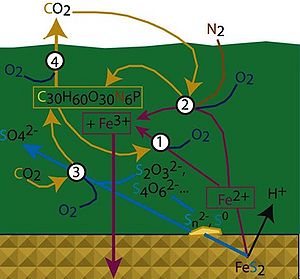
Ferroplasma spp. have been isolated across the world from heavy metal dominated/containing bioreactors or acid mine drainage (AMD) systems. These habitats are characterized by high metal concentrations (inorganic electron donors and acceptors), high pH that dissolve these inorganic compounds, medium to high temperatures and with some locations receiving sunlight. A AMD in particular has many microbial niches that vary in pH, temperature and ionic strength. However the nutrient limitation of an AMD allows only certain groups of organisms to grow. These high metal concentrations favor photo/chemo-autolithotrophs.
In an AMD, organisms that aerobically oxidize ferrous ion (with or without nitrogen and CO2 fixation), organisms that oxidize FeS2 to SO4- and those that oxidize organic carbon to CO2 coexists at different temperature ranges playing a role in the geochemical cycling of nutrients in the AMD (Figure 4). In the temperature range of growth of F. acidiphilum (mesophilic), Ferroplasma spp. aerobically oxidize ferrous ion to ferric ion (Figure 4 - marked 1). Organisms from the Leptospirillium spp. could also be involved in iron oxidation along with nitrogen and carbon fixation (Figure 4 -marked 2). Organisms of the Sulfobacillus spp. could be potentially involved in sulfur oxidation(Figure 4 -marked 3), while some eukaryotic organisms could be involved in the oxidation of organic carbon (Figure 4 -marked 4).
F. acidiphilum is not been shown to be pathogenic and its pathogenesis is irrelevant at present.
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
References
[6]
Author
Page authored by Nathan I. Johns and Indumathy Jayamani, students of Prof. Jay Lennon at Michigan State University.
