Giardia lamblia: Difference between revisions
(New page: {{Biorealm Genus}} ==Classification== ===Higher order taxa=== Domain; Phylum; Class; Order; family [Others may be used. Use [http://www.ncbi.nlm.nih.gov/Taxonomy/ NCBI] link to find] ...) |
No edit summary |
||
| (51 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
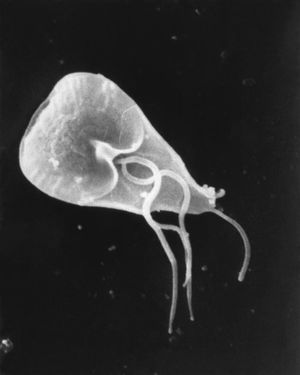
[[Image:8698_lores.jpg|thumb|Scanning electron micrograph (SEM) of Giardia lamblia trophozoite, showing external ultrastructural details.]] | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
| Line 5: | Line 7: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Domain | {|border="1" width="300" | ||
|- | |||
!width="150"|Domain | |||
|width="150"|Eukaryota | |||
|- | |||
! (Unspecified rank) | |||
| Diplomonadida group | |||
|- | |||
! (Unspecified rank) | |||
| Diplomonadida | |||
|- | |||
! Family | |||
| Hexamitidae | |||
|- | |||
! Sub-family | |||
| Giardiinae | |||
|- | |||
! Genus | |||
| Giardia | |||
|} | |||
===Species=== | ===Species=== | ||
{|border="1" width="300" | |||
|- | |||
!width="150"|Species | |||
|width="150"|Giardia <i>lamblia</i> | |||
|} | |||
Other names: ''Giardia intestinalis, Giardia duodenalis''<br/> | |||
{| | {| | ||
| height="10" bgcolor="# | | height="10" width="300" bgcolor="#FFCCFF" cellpadding="5" align="center" | | ||
''' | '''[http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=184922&lvl=3&keep=1&srchmode=1&unlock&lin=f NCBI Taxonomy]''' | ||
|} | |} | ||
'' | ==Description and significance== | ||
''Giardia lamblia'' is a flagellated, microaerophilic microorganism, first discovered by Van Leeuwenhoek in 1681, who found it in his own diarrheal stool. The ''G. lamblia'' trophozoite, vegetative, motile form of ''G. lamblia'' is pear-shaped and have unique morphology such as two identical nuclei, a ventral disc for adhesion to the host intestine, and flagella [see also [[#Trophozoite Structure]]]. The cyst is the reproductive form, and consists of a protective cyst wall as well as four nuclei. | |||
The genus ''Giardia'' has been isolated from more than 40 species. The species ''G. lamblia'' is known to infect human, mammals, reptiles, and birds, cows, sheeps and pigs, depending on the strain (Adam 2001). | |||
''G. lamblia'' is one of the major cause of waterborne diseases worldwide (CDC, 2004), and infection results in giardiasis (characterized by malabsorption and severe diarrhea). Giardia-induced intestinal infection is particularly severe in developing world, where giardiasis occurrence relates heavily to water source contamination. In the united states, ''G. lamblia'' has been found in both drinking and recreational water. Due to the high prevalence of giardiasis, ''G. lamblia'' is of significant interest in the clinical research community. However the pathogenic mechanisms are not completely understood. [See also [[#Pathology]]]. | |||
''G. lamblia'' is also significant in evolutionary biology. Due to its lack of mitochondria, ''G. lamblia'' is believed to be diverged from one of the earliest lineages of eukaryotes before the endosymbiotic relationship of mitochondria began (the kingdom name “Archezoa” has been proposed). However, this theory is currently under debate. Emerging proofs in genetics, for example, by comparison of the gene encoding valyl-tRNA synthetase (Hashimoto et al, 1998) and discovery of complex cellular machineries, (such as localized, mitochondria-like electron-transport machinery, (Lloyd, 2002)) suggest they may be more advanced organisms, who were once mitochondria-bearing but lost them ever since. Sequencing of the complete genome of ''G. lambdia'' is currently in progress, but results so far have already provided much insight into its evolutionary history. | |||
==Genome structure== | ==Genome structure== | ||
''G. lamblia'' possess two equal-sized nuclei, each contains an entire genome of equal sizes (Yu et al, 2002). Both nuclei are transcriptionally active and are replicated simultaneously during cell division. (Adam, 2001). | |||
The ''G. lamblia'' genome consists of 1.2 million base pairs, distributed among five linear chromosomes, each flanked by the telomere sequence (5’TAGGG3’) comparable to other eukaryotes (Le Blancq et al, 1991). Chromosome packing is done by core histones, but the mechanism is slightly different from other eukayotes (Yee et al, 2007). [See also [[#Current Research]]]. | |||
The genome has an average GC content of 46% ([http://www.mbl.edu/Giardia Giardia lamblia genome project]). Complete mapping of the ''G. lamblia'' genome (WB strain) is in progress (95% complete, as of 2007 August), using shotgun sequencing. So far 6488 open reading frames (ORF) are predicted, of which 4746 are transcribed. | |||
Nevertheless, studies have been conducted based on the partial genome. Its comparison among other eukaryotic species has provided new perspective in the evolution of this species. | |||
Studies by comparing upstream sequence to cytoskeletal protein-coding genes have found several consensus sequence, for example, an AT-rich region 5’AATTAAAAA3’ found between –30 and –70 upstream. These sequences have been proposed as promoter regions for ''G. lamblia'', although they are relatively shorter than that of other eukaryotes. The AT-rich region shown above has been found to be essential for transcription. In addition, recent studies have shown that ''G. lamblia'' promoters, in particular, the AT-rich, “TATA”-like regions, cause the production of sterile anti-sense transcripts (Teodorvic et al, 2007). | |||
Genes coding for homologs of mitochondrial proteins such as heat shock protein 70 and chaperonin 60 (cpn60) have been identified in the ''G. lamblia'' genome (Roger et al, 1998) and the expression has been found throughout the entire life cycle except during excystation. This suggests that the Gardia was not pre-mitochondrial, but incorporated the mitochondrial ancestor but lost them ever since. In the same context, genes encoding for valy-tRNA synthetase (ValRS), which are believed to be originated from the mitochondria, have also been found in the ''G. lamblia'' genome (Hashimoto et al, 1998). | |||
==Cell structure== | |||
===Trophozoite Structure=== | |||
The vegetative form of ''G. lamblia'', trophozoites, are 12 to 15 μm in length, and 5 to 9 μm in width. They have unique characteristics in morphology: a binuclear structure (each containing a complete set of genome (Yu, et al, 2002), four pairs of flagella, a ventral disc, and a median body. The two nuclei are almost identical, have no visible nucleoli, and are arranged in symmetric fashion. | |||
The cytoskeleton of trophozoites, in particular the ventral disc, is believed to play a major role in its attachment to the host intestine (Adam, 2001). Proteins that are found on exclusively on the ventral disc, such as actinin, alpha-actinin, myosin and tropomysin (Feely et al, 1982), as well as lectin (Farthing et al, 1986), have been proposed as biochemical agents involved in attachment. The four pairs of flagella are located on the anterior r, posterior, caudal and ventral side of the organism. Each is composed of 11 microtubules (including two core microtubules). | |||
Centrin, a cytoskeletal protein, has been found in trophozoites. Immunoflorescence studies have revealed its presence in all microtubular systems in trophozoites including the flagella, median bodies, basal bodies, as well as the adhesive disc (Corrêa et al, 2004). This suggests that the Giardia centrin could be a Microtubule-associated protein, and it might be involved in motility and adhesion. | |||
Specialized membrane structures for electron transport and generation of membrane potential, as seen in mitochondria (Lloyd 2002), have also been discovered on the trophozoite cytoplasmic membrane, suggesting their evolutionary relationship with the mitochondria. | |||
Endoplasmic recticulum- (ER) or Golgi-like structures, essential for protein trafficking in eukaryotes, have not been observed in ''G. lamblia'' trophozoites using the microscope. However, common ER-bound proteins such as chaperonin BiP have been identified in an extensive membrane-bound system (Soltys et al, 1996). This suggests the presence of the ER-like structure, with functionalities similar to those in other eukaryotes. The presence of ARF protein, required for vesicle budding, also suggests the presence of Golgi-like structure (Lujan et al, 1995), although further investigation is required. | |||
===Cyst Structure=== | |||
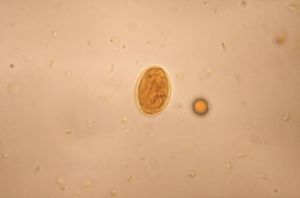
[[Image:PHIL_3742_lores.jpg|thumb|Micrograph of Giardia lamblia cyst using an iodine staining technique.]] | |||
The ''G. lamblia'' cyst is 7 to 10 um in diameter, and contains four nuclei. The cyst is covered by a 0.3~0.5um-thick cyst wall. The cyst wall is composed of two layers: outer filamentous layer and an inner membranous layer (further composed with two membranes). (Adam, 2001) Upon excystation, one cyst matures into two trophozoites. | |||
==Metabolism== | |||
The cyst form has a reduced metabolic rate compared to the trophozoites (approximately 10~20% of the trophozoites), and it is stimulated by ethanol (Paget et al, 1989). | |||
The ''G. lamblia'' trophozoite are microaerophilic, lacks of mitochondria and relies on cytochrome-mediated oxidative phosphorylation. (Adam, 2001). They can perform aerobic and anaerobic metabolism depending on environmental oxygen concentration, although they predominately rely on fermentation. In addition, fermentation is carried out even in the presence of oxygen. | |||
===Aerobic and Anaerobic Metabolism=== | |||
The Metabolic activity of the trophozoite varies on concentration of oxygen and glucose present. When oxygen is absent, glucose metabolism is predominant, and alanine is the major product. In the presence of oxygen, Alanine production is inhibited (Papanastasiou et al, 1997). When oxygen reaches a concentration of 46 uM, CO2 and acetate are the major product of metabolism. | |||
Common enzymes for oxygen detoxification, such as superoxide dismutase, catalase, peroxidase, have not been found in the ''G. lamblia'' trophozoite (Brown, 1995). In ''G. lamblia'', oxidative stress management is believed to be accomplished by a thioredoxin reductase class of disulphide reductase, which uses cystine as primary electron acceptor (Brown et al., 1996b). Recent findings have revealed that the cytoplasmic enzyme NAD(P)H menadione oxidoreductase (DT-diaphorase) also play a role in oxidative stress management (Sanchez et al, 2001), and can significantly enhance growth upon overexpression (Lei et al, 2006). | |||
===Carbohydrate metabolism=== | |||
Glucose is required for trophozoite growth, but not essential. It contributes to the major source of energy derived from carbohydrates. The glucose metabolism pathway of ''G. lamblia'' is similar to other eukaryotes, except in minor aspects. For example, the conversion from Fructose-6-Phosphate to Fructose-1,6-bisphosphate is irreversible in most prokaryotes and eukaryotes, catalyzed by a phosphofructokinase, requires ATP and is regulated. However in ''G. lamblia'', this process is not regulated. And the phosphofructokinase is not ATP-dependent, instead, pyrophosphate-dependent (Adam 2001, Michels et al, 2006). | |||
For energy storage, there has been evidence that trophozoite uses glycogen as an energy reserve (Ladeira et al, 2005). | |||
===Amino acid metabolism=== | |||
''G. lamblia'' is only capable of de novo synthesis of Alanine (for energy metabolism), and Valine. It relies on savaging all other amino acids from the host intestine. | |||
Nevertheless, amino acid is an important energy source of energy for the species. The major amino acid pathways use arginine and aspartate (Adam, 2000). The former pathway uses arginine dihydrolase, and is present in many prokaryotes but only two eukaryotes to date. This pathway converts arginine into ammonia and carbon dioxide, and generate ATP by substrate-level phosphorylation. Ornithine, a product, is used for a membrane antiport for importation of extracellular arginine (Edwards, 1992). | |||
Amino acids imported are also used for osmoregulation and protection. When environmental osmolarity drops, intracellular alanine is excreted (Edwards et al, 1993 and Nygaard et al, 1994). The antiport involved also transport L-serine, glycine, L-threonine, L-glutamine, and L-asparagine. Cysteine is found to play a role in protection against oxygen toxicity. Cysteine is the major source of free-thiol groups on variable surface proteins (VSP) on the trophozoite, and this has been shown by using radio-labeled cysteine (Aggarwal et al, 1989) | |||
== | ===Hydrogen metabolism=== | ||
''G. lamblia'' is found to generate low amount of hydrogen (2 nmol/min/10<sub>E</sub>7 organisms) under strict anaerobic conditions (Llyod et al. 2002). No identifiable hydrogenosomes are found, although they are often found in other hydrogen-producing species such as the trichomonads). | |||
==Ecology== | ==Ecology== | ||
The ''G. lamblia'' life cycle consists of two stages: the cyst and trophozoites. The cyst is highly resistance against chlorination, ozonolysis, and can survive in cold water between 4~8 degrees Celsius (Ali et al, 2003). It is often found in contaminated water and feces of infected individual. The trophozoite form is the vegetative form of ''G. lamblia'', and is found in the small intestine of infected individuals. It may also be found in their feces. The trophozoite is almost exclusively found in the human intestine, its primary niche. | |||
===Acquisition and Excystation=== | |||
Acuisition of Giardia is primarily through the cyst form, in the fecal-oral route or, in unindustrialized regions, through contaminated water. The cyst is protected against the host’s gastric acid with its cyst wall. Studies have suggested that its passage through the stomach of the host, provided an acidic environment that contributes to initiation of excystation, transformation from the cyst form to trophozoite. (Elmendorf et al, 2003 and Adam 2001) Excystation is also facilitated by pancreatic protease, and cysteine protease CP2. In addition, evidence have been gathered to show that excystation may be inhibited by antibody to the cyst wall, calmodulin antagonists TFP and W7 (Bernal et al, 1998), and by wheat germ agglutinin (Meng et al, 1996). | |||
===Attachment and Reproduction=== | |||
The trophozoite adheres to the wall of the small intestine using its ventral disc, and replicates within the lumen. Attachment was thought be accomplished by the hydrodynamic force generated under the disc using the ventral flagella (Holberton et al, 1974) Another proposed mechanisms for attachment involve a surface lectin, found on the ventral disc of the trophozoites, that binds to mannose. (Farthing et al, 1986). | |||
Reproduction and encystations (formation of cyst) are carried out in the host mid-mid-jejunum (small intestine). The metabolic activity of the trophozoite may cause diarrhea, malabsorption and weight loss- a condition known as Giardiasis [see Pathology for details]. In some patients, no symptom may develop. The reason of this is unknown, but it is believed that the parasite strain and host factors may play a role (Thomson, 2000) | |||
===Encystation=== | |||
Encystation is observable under the microscope, and detailed mechanisms have been characterized by biochemical methods. | |||
Encystation occurs at mild alkalotic pH of 7.8 with the presence of fatty acid conjugated with bile salts (Gillin et al, 1988). In vitro studies have shown that encystations is induced by cholesterol and exposure of bile salts and fatty acids (Kane et al, 1991 and Gillin et al, 1996), but this remains controversial. | |||
There are two phases of encystation. In the early phase, cell wall proteins are synthesized and transported to the periphery. Increased cell wall protein synthesis renders a Golgi-like structure readily visible with electron microscopy (Reiner et al, 1990). The proteins, containing a leucine-rich signature, are redirected to Encystation-specific transport vesicles (ESV), visible under light microscopy (Hehl et al, 2000). | |||
The late phase involves the assembly of the cyst wall and morphological change. The assembly begins with cyst wall filaments, then the filamentous layer of the cell wall (Adam, 2001). During the course of late-phase encystations, the trophozoite loses motility. Eventually a non-adhering cyst enclosing two physically joined trophozoites with four nuclei results. | |||
==Pathology== | ==Pathology== | ||
Giardiasis is the clinical manifestation of ''G. lamblia'' infection. It is characterized by severe diarrhea, malnutrition weight loss, and slight intestinal epithelial injury. The complete pathogenesis is no well known, but several theories exist. | |||
The disruption of the small intestine epithelium is proposed to be caused by induced apoptosis (Chin et al, 2002). Recently this theory was proved in vitro. Both intrinsic and extrinsic apoptotic pathways on enterocytes were observed. In addition, Bcl-2 was downregulated, and caspase-3 action was assayed (Panaro et al, 2007). However this theory appears to be strain-specific. It has also been shown that ''G. lamblia'' increases permeability of single-layered duodenal epithelium in vitro, by phosphorylating F-actin and zonaoccludens in enterocytes (Scott et al, 2002). | |||
In addition, immmune evasion of ''G. lamblia'' is achieved through variation in expression of variant-specific surface proteins (VSP), a family of variable antigenic makers of unknown function (Singer et al, 2000). Selective pressure on parasite is both positive and negative, and is dependent on the VSP. Host-specific factors may also involved. | |||
Although the most prominent clinical manifestations of Giardiasis are diarrhea and malabsorption, detailed mechanisms are not completely characterized. However, colonization of the microbe appears cause microvillus shortening (Scott et al, 2000), villous flattening or atrophy (Williamson et al., 2000). It is thought that these mechanisms may work synergistically with other mechanisms such as inbhition of disaccharase and protease to produce the overall symptoms (Muller et al, 2005). | |||
Recently, a study has shown that in Giardiasis, diarreha is a causative combination of leak flux, malabsorption and malsecretion: downregulation of tight junction protein claudin 1, and epithelial apotosis causes failure of sodium-dependent glucose absorption, which results in active chloride ion secretion. Consequently, water enters the lumen, eliciting diarrhea (Troeger, 2007). | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
None has been documented to date. | |||
==Current Research== | ==Current Research== | ||
''G. lamblia'' is rigorously under research for its significance in epidemiology, clinical medicine and evolutionary biology. | |||
* Due to the widespread of waterborne disease casued by ''G. lamblia'', it is one major field of study for epidemiologists. Poereira et al evaluated the prevalence of Giardiasis among hospitalized children suffering from diarrhea in Goiânia, Brazil and determined several risk factors for ''G. lamblia'' infection (Pereira et al, 2007). These risk factors include: number of children in the household, food hygiene, presence of cats and attendance to day-care centers. Similar studies are currently held by the CDC and other government agencies worldwide (CDC, 2004). Identification of such risk factors allow enable policy makers to target the disease in terms of of legislation and public health services. | |||
* Drug resistance of ''G. lamblia'' (strain WB C6 clones) against nitazoxanide and metronidazole has been studied (Muller et al, 2007). The transcription of several proteins involved in the resistance formation, such as pyruvate oxireductases 1 and 2, nitroreductase, VSPs, and protein disulfide isomerases 2 and 4, were quantified and correlated with each strain. And it was found that each strain, synthesis of a particular set of proteins was inhibited. Moreover, it was found that VSP expression was altered dramatically by the drugs. The same researchers are planning to combine genomic and proteonomic methods to understand cellular mechanisms in details, and their role in drug resistance in the parasite. | |||
* A study of the genetic structure of ''G. lamblia'' (Yee et al, 2007), has located genes for core histones H2a, H2b, H3 and H4 but not the gene for linker histone H1. Sequence comparison shows similar sequence in both coding and 5’ non-coding regions. This is indicative of recent gene-duplication or conversion. This study suggests an alternative mechanism of gene-compacting in ''G. lamblia'', which might be accomplished without the H1 histone, to optimize the transcription of the gene-rich DNA of the species. Such understanding would be fundemental in knowing the gene expression in this species. | |||
* The [http://www.mbl.edu/Giardia Giardia lamblia genome project] at the Marine Biological Laboratory in Masshuttes, in which shotgun sequencing is used to obtain the full genome of ''G. lamblia'', is currently under way. Annotated genome assembly is released regularly and is made available to researchers worldwide. The analysis of gene function is done currently in collaboration with Dr. Frances Gillin at UCSD molecular pathology. This study is of great significance in evolutionary biology, as it will provide insight into the history of ''G. lamblia': its relation with the mitochondria, and its role in serving as the bridge between prokaryotes and eukaryotes. The full genome will also assist researchers in identifying possible genes that codes for toxins and possibly derive drugs that could inhibit the parasite. | |||
==References== | ==References== | ||
# Adam, RD, & Adam. 2001. Biology of Giardia lamblia. Clinical microbiology reviews, 14(3), 447-. | |||
# Aggarwal, A., J. W. Merritt, Jr., and T. E. Nash. 1989. Cysteine-rich variant surface proteins of Giardia lamblia. Mol. Biochem. Parasitol. 32:39–47. | |||
# Ali, SA, & Hill, DR. 2003. Giardia intestinalis. Current opinion in infectious diseases, 16(5), 453-60. | |||
# Bernal, R. M., R. Tovar, J. I. Santos, and M. L. Munoz. 1998. Possible role of calmodulin in excystation of Giardia lamblia. Parasitol. Res. 84:687–693. | |||
# Brown, D.M., Upcroft, J.A., Upcroft, P. 1995. Free radical detoxification in Giardia duodenalis. Mol Biochem Parasitol 72: 47–56. | |||
# Brown, D.M., Upcroft, J.A., Upcroft, P. 1996b. A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol Biochem Parasitol 83: 211–220. | |||
# Center for Disease Control, [http://www.cdc.gov/ncidod/dpd/parasites/giardiasis/2004_PDF_Giardiasis.pdf Giardia Infection Fact Sheet]. 2004. Retrieved 2007 August 29. | |||
# Chin AC, Teoh DA, Scott KG, et al. 2002. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun 2002; 70:3673–3680. | |||
# Corrêa, G, Morgado-Diaz, JA, & Benchimol, M., 2004. Centrin in Giardia lamblia - ultrastructural localization. FEMS microbiology letters, 233(1), 91-6. | |||
# Edwards, M. R., L. A. Knodler, J. R. Wilson, and P. J. Schofield. 1993. The transport and metabolism of alanine by Giardia intestinalis. Mol. Biochem. Parasitol. 61:49–57. | |||
# Edwards, M. R., P. J. Schofield, W. J. O’Sullivan, and M. Costello. 1992. Arginine metabolism during culture of Giardia intestinalis. Mol. Biochem. Parasitol. 53:97–103. | |||
# Elmendorf, HG, Dawson, SC, & McCaffery, JM. 2003. The cytoskeleton of Giardia lamblia. International Journal for Parasitology, 33(1), 3-28. | |||
# Farthing MJG, Pereira MEA, Keusch GT 1986. Description and characterization of a surface lectin from Giardia lamblia. Infect Immunol 1986;51:661-7. | |||
# Feely, D. E., J. V. Schollmeyer, and S. L. Erlandsen. 1982. Giardia spp.: Distribution of contractile proteins in the attachment organelle. Exp. Parasitol. 53:145–154. | |||
# Gillin, F. D., D. S. Reiner, and S. E. Boucher. 1988. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infect. Immun. 56:705–707. | |||
# Gillin, F. D., Reiner, D. S., and McCaffery, J. M. 1996. Cell biology of the primitive eukaryote Giardia lamblia. Annu. Rev. Microbiol. 50, 679-705 | |||
# Hashimoto T, Sanchez LB, Shirakura T, Muller M, Hasegawa M. 1998. Secondary absence of mitochondria in Giardia lamblia and Trichomonas vaginalis revealed by valyl-tRNA synthetase phylogeny. Proc Natl Acad Sci USA 1998;95:6860-5. | |||
# Hashimoto, T., L. B. Sanchez, T. Shirakura, M. Muller, and M. Hasegawa. 1998. Secondary absence of mitochondria in Giardia lamblia and Trichomonas vaginalis revealed by valyl-tRNA synthetase phylogeny. Proc. Natl. Acad. Sci. USA 95:6860–6865. | |||
# Hehl, A. B., M. Marti, and P. Kohler. 2000. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol. Biol. Cell 11:1789–1800. | |||
# Holberton DV and Marshall J. 1995. Analysis of consensus sequence patterns in Giardia cytoskeleton gene promoters. Nucleic Acids Res 1995;23:2945-53. Link. | |||
# Holberton DV. 1974. Attachment of Giardia-a hydrodynamic model based on flagellar activity. J Exp Biol 1974;60:207-21. | |||
# Kane, A. V., Ward, H. D., Keusch, G. T., and Pereira, M. E. A. 1991. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J. Parasitol. 77, 974-981 | |||
# Ladeira, RB, Freitas, MA, Silva, EF, et al. 2005. Glycogen as a carbohydrate energy reserve in trophozoites of Giardia lamblia. Parasitology research, 96(6), 418-21. | |||
# Le Blancq, S. M., R. S. Kase, and L. H. Van der Ploeg. 1991. Analysis of a Giardia lamblia rRNA encoding telomere with [TAGGG]n as the telomere repeat. Nucleic Acids Res. 19:5790–5790. | |||
# Lei Li, Ching C. Wang 2006. A likely molecular basis of the susceptibility of Giardia lamblia Towards oxygen Molecular Microbiology 59 (1), 202–211. | |||
# Lloyd, D, Harris, JC, Maroulis, S, et al. 2002. The "primitive" microaerophile Giardia intestinalis (syn. lamblia, duodenalis) has specialized membranes with electron transport and membrane-potential-generating functions. Microbiology, 148(Pt 5), 1349-54. | |||
# Lujan, H. D., A. Marotta, M. R. Mowatt, N. Sciaky, J. Lippincott-Schwartz, and T. E. Nash. 1995. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 270: 4612–4618. | |||
# Meng, T. C., M. L. Hetsko, and F. D. Gillin. 1996. Inhibition of Giardia lamblia excystation by antibodies against cyst walls and by wheat germ agglutinin. Infect. Immun. 64:2151–2157. | |||
# Michels, PA, & Rigden, DJ. 2006. Evolutionary analysis of fructose 2,6-bisphosphate metabolism. IUBMB life, 58(3), 133-41. | |||
# Muller N, Allmen NV. 2005. Recent insights into the mucosal reactions associated with Giardia lamblia infections. International Journal for Parasitology 35 (2005) 1339–1347 | |||
# Muller, J, & Muller. 2007. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. The journal of antimicrobial chemotherapy, 60(2), 280-. | |||
# Nygaard, T., C. C. Bennett, G. Grossman, M. R. Edwards, and P. J. Schofield. 1994. Efflux of alanine by Giardia intestinalis. Mol. Biochem. Parasitol. 64:145–152. | |||
# Paget, T. A., E. L. Jarroll, P. Manning, D. G. Lindmark, and D. Lloyd. 1989. Respiration in the cysts and trophozoites of Giardia muris. J. Gen. Microbiol. 135:145–154. | |||
# Panaro MA, Cianciulli A, Mitolo V, Mitolo CI, Acquafredda A, Brandonisio O, Cavallo P. 2007. [http://dx.doi.org/10.1111/j.1574-695X.2007.00304.x Caspase-dependent apoptosis of the HCT-8 epithelial cell line induced by the parasite Giardia intestinalis]. FEMS Immunol Med Microbiol. 2007 Aug 20; | |||
# Papanastasiou, P., T. Bruderer, Y. Li, C. Bommeli, and P. Kohler. 1997. Primary structure and biochemical properties of a variant-specific surface protein of Giardia. Mol. Biochem. Parasitol. 86:13–27. | |||
# Pereira, MGC, ATWILL, ER and BARBOSA, AP. 2007. Prevalence and associated risk factors for Giardia lamblia infection among children hospitalized for diarrhea in Goiânia, Goiás State, Brazil. Rev. Inst. Med. trop. S. Paulo, May/June 2007, vol.49, no.3, p.139-145 | |||
# Reiner, D. S., M. McCaffery, and F. D. Gillin. 1990. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur. J. Cell Biol. 53:142–153. | |||
# Roger AJ, Svard SG, Tovar J et al. 1998. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. 1998. Proc Natl Acad Sci USA;95:229-34. | |||
# Sanchez, L.B., Elmendorf, H., Nash, T.E., Muller, M. 2001. NAD(P)H:menadione oxidoreductase of the amitochondriate eukaryote Giardia lamblia: a simpler homologue of the vertebrate enzyme. Microbiology 147: 561–570. | |||
# Scott KG, Meddings JB, Kirk DR, et al. 2002. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123:1179–1190. | |||
# Scott, K.G., Logan, M.R., Klammer, G.M., Teoh, D.A., Buret, A.G., 2000. Jejunal brush border microvillous alterations in Giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infect. Immun. 68, 3412–3418. | |||
# Singer SM, Elmendorf HG, Conrad JT, Nash TE. 2000. Biological Selection of Variant-Specific Surface Proteins in Giardia lamblia. J. Infectious Diseases 2001;183:119-124 | |||
# Soltys, B. J., M. Falah, and R. S. Gupta. 1996. Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to Bip. J. Cell Sci. 109:1909–1917. | |||
# Teodorovic, S. Walls, CD. and Elmendorf, HG. 2007. Bidirectional transcription is an inherent feature of Giardia lamblia promoters and contributes to an abundance of sterile antisense transcripts throughout the genome. Nucleic Acids Research, 2007, Vol. 35, No. 8 2544-2553. Link. | |||
# Thompson, R.C.A., 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30, pp. 1259–1267. | |||
# Troeger, H, Epple, HJ, Schneider, T, et al., 2007. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut, 56(3), 328-35. | |||
# Yee, J, Tang, A, Lau, WL, et al. 2007. Core histone genes of Giardia intestinalis: genomic organization, promoter structure, and expression. BMC molecular biology, 8, 26-. | |||
# Yu, LZ. Birky, C. Willia, Jr, Adam, RD. 2002. The Two Nuclei of Giardia Each Have Complete Copies of the Genome and Are Partitioned Equationally at Cytokinesis. EUKARYOTIC CELL, Apr. 2002, p. 191–199 a | |||
Edited by student [mailto:d.lau@none D.Lau] of [mailto:ralarsen@ucsd.edu Rachel Larsen] | |||
Edited by | Edited by KLB | ||
Latest revision as of 15:42, 1 July 2011
A Microbial Biorealm page on the genus Giardia lamblia
Classification
Higher order taxa
| Domain | Eukaryota |
|---|---|
| (Unspecified rank) | Diplomonadida group |
| (Unspecified rank) | Diplomonadida |
| Family | Hexamitidae |
| Sub-family | Giardiinae |
| Genus | Giardia |
Species
| Species | Giardia lamblia |
|---|
Other names: Giardia intestinalis, Giardia duodenalis
Description and significance
Giardia lamblia is a flagellated, microaerophilic microorganism, first discovered by Van Leeuwenhoek in 1681, who found it in his own diarrheal stool. The G. lamblia trophozoite, vegetative, motile form of G. lamblia is pear-shaped and have unique morphology such as two identical nuclei, a ventral disc for adhesion to the host intestine, and flagella [see also #Trophozoite Structure]. The cyst is the reproductive form, and consists of a protective cyst wall as well as four nuclei.
The genus Giardia has been isolated from more than 40 species. The species G. lamblia is known to infect human, mammals, reptiles, and birds, cows, sheeps and pigs, depending on the strain (Adam 2001).
G. lamblia is one of the major cause of waterborne diseases worldwide (CDC, 2004), and infection results in giardiasis (characterized by malabsorption and severe diarrhea). Giardia-induced intestinal infection is particularly severe in developing world, where giardiasis occurrence relates heavily to water source contamination. In the united states, G. lamblia has been found in both drinking and recreational water. Due to the high prevalence of giardiasis, G. lamblia is of significant interest in the clinical research community. However the pathogenic mechanisms are not completely understood. [See also #Pathology].
G. lamblia is also significant in evolutionary biology. Due to its lack of mitochondria, G. lamblia is believed to be diverged from one of the earliest lineages of eukaryotes before the endosymbiotic relationship of mitochondria began (the kingdom name “Archezoa” has been proposed). However, this theory is currently under debate. Emerging proofs in genetics, for example, by comparison of the gene encoding valyl-tRNA synthetase (Hashimoto et al, 1998) and discovery of complex cellular machineries, (such as localized, mitochondria-like electron-transport machinery, (Lloyd, 2002)) suggest they may be more advanced organisms, who were once mitochondria-bearing but lost them ever since. Sequencing of the complete genome of G. lambdia is currently in progress, but results so far have already provided much insight into its evolutionary history.
Genome structure
G. lamblia possess two equal-sized nuclei, each contains an entire genome of equal sizes (Yu et al, 2002). Both nuclei are transcriptionally active and are replicated simultaneously during cell division. (Adam, 2001).
The G. lamblia genome consists of 1.2 million base pairs, distributed among five linear chromosomes, each flanked by the telomere sequence (5’TAGGG3’) comparable to other eukaryotes (Le Blancq et al, 1991). Chromosome packing is done by core histones, but the mechanism is slightly different from other eukayotes (Yee et al, 2007). [See also #Current Research].
The genome has an average GC content of 46% (Giardia lamblia genome project). Complete mapping of the G. lamblia genome (WB strain) is in progress (95% complete, as of 2007 August), using shotgun sequencing. So far 6488 open reading frames (ORF) are predicted, of which 4746 are transcribed.
Nevertheless, studies have been conducted based on the partial genome. Its comparison among other eukaryotic species has provided new perspective in the evolution of this species.
Studies by comparing upstream sequence to cytoskeletal protein-coding genes have found several consensus sequence, for example, an AT-rich region 5’AATTAAAAA3’ found between –30 and –70 upstream. These sequences have been proposed as promoter regions for G. lamblia, although they are relatively shorter than that of other eukaryotes. The AT-rich region shown above has been found to be essential for transcription. In addition, recent studies have shown that G. lamblia promoters, in particular, the AT-rich, “TATA”-like regions, cause the production of sterile anti-sense transcripts (Teodorvic et al, 2007).
Genes coding for homologs of mitochondrial proteins such as heat shock protein 70 and chaperonin 60 (cpn60) have been identified in the G. lamblia genome (Roger et al, 1998) and the expression has been found throughout the entire life cycle except during excystation. This suggests that the Gardia was not pre-mitochondrial, but incorporated the mitochondrial ancestor but lost them ever since. In the same context, genes encoding for valy-tRNA synthetase (ValRS), which are believed to be originated from the mitochondria, have also been found in the G. lamblia genome (Hashimoto et al, 1998).
Cell structure
Trophozoite Structure
The vegetative form of G. lamblia, trophozoites, are 12 to 15 μm in length, and 5 to 9 μm in width. They have unique characteristics in morphology: a binuclear structure (each containing a complete set of genome (Yu, et al, 2002), four pairs of flagella, a ventral disc, and a median body. The two nuclei are almost identical, have no visible nucleoli, and are arranged in symmetric fashion.
The cytoskeleton of trophozoites, in particular the ventral disc, is believed to play a major role in its attachment to the host intestine (Adam, 2001). Proteins that are found on exclusively on the ventral disc, such as actinin, alpha-actinin, myosin and tropomysin (Feely et al, 1982), as well as lectin (Farthing et al, 1986), have been proposed as biochemical agents involved in attachment. The four pairs of flagella are located on the anterior r, posterior, caudal and ventral side of the organism. Each is composed of 11 microtubules (including two core microtubules).
Centrin, a cytoskeletal protein, has been found in trophozoites. Immunoflorescence studies have revealed its presence in all microtubular systems in trophozoites including the flagella, median bodies, basal bodies, as well as the adhesive disc (Corrêa et al, 2004). This suggests that the Giardia centrin could be a Microtubule-associated protein, and it might be involved in motility and adhesion.
Specialized membrane structures for electron transport and generation of membrane potential, as seen in mitochondria (Lloyd 2002), have also been discovered on the trophozoite cytoplasmic membrane, suggesting their evolutionary relationship with the mitochondria.
Endoplasmic recticulum- (ER) or Golgi-like structures, essential for protein trafficking in eukaryotes, have not been observed in G. lamblia trophozoites using the microscope. However, common ER-bound proteins such as chaperonin BiP have been identified in an extensive membrane-bound system (Soltys et al, 1996). This suggests the presence of the ER-like structure, with functionalities similar to those in other eukaryotes. The presence of ARF protein, required for vesicle budding, also suggests the presence of Golgi-like structure (Lujan et al, 1995), although further investigation is required.
Cyst Structure
The G. lamblia cyst is 7 to 10 um in diameter, and contains four nuclei. The cyst is covered by a 0.3~0.5um-thick cyst wall. The cyst wall is composed of two layers: outer filamentous layer and an inner membranous layer (further composed with two membranes). (Adam, 2001) Upon excystation, one cyst matures into two trophozoites.
Metabolism
The cyst form has a reduced metabolic rate compared to the trophozoites (approximately 10~20% of the trophozoites), and it is stimulated by ethanol (Paget et al, 1989).
The G. lamblia trophozoite are microaerophilic, lacks of mitochondria and relies on cytochrome-mediated oxidative phosphorylation. (Adam, 2001). They can perform aerobic and anaerobic metabolism depending on environmental oxygen concentration, although they predominately rely on fermentation. In addition, fermentation is carried out even in the presence of oxygen.
Aerobic and Anaerobic Metabolism
The Metabolic activity of the trophozoite varies on concentration of oxygen and glucose present. When oxygen is absent, glucose metabolism is predominant, and alanine is the major product. In the presence of oxygen, Alanine production is inhibited (Papanastasiou et al, 1997). When oxygen reaches a concentration of 46 uM, CO2 and acetate are the major product of metabolism.
Common enzymes for oxygen detoxification, such as superoxide dismutase, catalase, peroxidase, have not been found in the G. lamblia trophozoite (Brown, 1995). In G. lamblia, oxidative stress management is believed to be accomplished by a thioredoxin reductase class of disulphide reductase, which uses cystine as primary electron acceptor (Brown et al., 1996b). Recent findings have revealed that the cytoplasmic enzyme NAD(P)H menadione oxidoreductase (DT-diaphorase) also play a role in oxidative stress management (Sanchez et al, 2001), and can significantly enhance growth upon overexpression (Lei et al, 2006).
Carbohydrate metabolism
Glucose is required for trophozoite growth, but not essential. It contributes to the major source of energy derived from carbohydrates. The glucose metabolism pathway of G. lamblia is similar to other eukaryotes, except in minor aspects. For example, the conversion from Fructose-6-Phosphate to Fructose-1,6-bisphosphate is irreversible in most prokaryotes and eukaryotes, catalyzed by a phosphofructokinase, requires ATP and is regulated. However in G. lamblia, this process is not regulated. And the phosphofructokinase is not ATP-dependent, instead, pyrophosphate-dependent (Adam 2001, Michels et al, 2006).
For energy storage, there has been evidence that trophozoite uses glycogen as an energy reserve (Ladeira et al, 2005).
Amino acid metabolism
G. lamblia is only capable of de novo synthesis of Alanine (for energy metabolism), and Valine. It relies on savaging all other amino acids from the host intestine.
Nevertheless, amino acid is an important energy source of energy for the species. The major amino acid pathways use arginine and aspartate (Adam, 2000). The former pathway uses arginine dihydrolase, and is present in many prokaryotes but only two eukaryotes to date. This pathway converts arginine into ammonia and carbon dioxide, and generate ATP by substrate-level phosphorylation. Ornithine, a product, is used for a membrane antiport for importation of extracellular arginine (Edwards, 1992).
Amino acids imported are also used for osmoregulation and protection. When environmental osmolarity drops, intracellular alanine is excreted (Edwards et al, 1993 and Nygaard et al, 1994). The antiport involved also transport L-serine, glycine, L-threonine, L-glutamine, and L-asparagine. Cysteine is found to play a role in protection against oxygen toxicity. Cysteine is the major source of free-thiol groups on variable surface proteins (VSP) on the trophozoite, and this has been shown by using radio-labeled cysteine (Aggarwal et al, 1989)
Hydrogen metabolism
G. lamblia is found to generate low amount of hydrogen (2 nmol/min/10E7 organisms) under strict anaerobic conditions (Llyod et al. 2002). No identifiable hydrogenosomes are found, although they are often found in other hydrogen-producing species such as the trichomonads).
Ecology
The G. lamblia life cycle consists of two stages: the cyst and trophozoites. The cyst is highly resistance against chlorination, ozonolysis, and can survive in cold water between 4~8 degrees Celsius (Ali et al, 2003). It is often found in contaminated water and feces of infected individual. The trophozoite form is the vegetative form of G. lamblia, and is found in the small intestine of infected individuals. It may also be found in their feces. The trophozoite is almost exclusively found in the human intestine, its primary niche.
Acquisition and Excystation
Acuisition of Giardia is primarily through the cyst form, in the fecal-oral route or, in unindustrialized regions, through contaminated water. The cyst is protected against the host’s gastric acid with its cyst wall. Studies have suggested that its passage through the stomach of the host, provided an acidic environment that contributes to initiation of excystation, transformation from the cyst form to trophozoite. (Elmendorf et al, 2003 and Adam 2001) Excystation is also facilitated by pancreatic protease, and cysteine protease CP2. In addition, evidence have been gathered to show that excystation may be inhibited by antibody to the cyst wall, calmodulin antagonists TFP and W7 (Bernal et al, 1998), and by wheat germ agglutinin (Meng et al, 1996).
Attachment and Reproduction
The trophozoite adheres to the wall of the small intestine using its ventral disc, and replicates within the lumen. Attachment was thought be accomplished by the hydrodynamic force generated under the disc using the ventral flagella (Holberton et al, 1974) Another proposed mechanisms for attachment involve a surface lectin, found on the ventral disc of the trophozoites, that binds to mannose. (Farthing et al, 1986).
Reproduction and encystations (formation of cyst) are carried out in the host mid-mid-jejunum (small intestine). The metabolic activity of the trophozoite may cause diarrhea, malabsorption and weight loss- a condition known as Giardiasis [see Pathology for details]. In some patients, no symptom may develop. The reason of this is unknown, but it is believed that the parasite strain and host factors may play a role (Thomson, 2000)
Encystation
Encystation is observable under the microscope, and detailed mechanisms have been characterized by biochemical methods.
Encystation occurs at mild alkalotic pH of 7.8 with the presence of fatty acid conjugated with bile salts (Gillin et al, 1988). In vitro studies have shown that encystations is induced by cholesterol and exposure of bile salts and fatty acids (Kane et al, 1991 and Gillin et al, 1996), but this remains controversial.
There are two phases of encystation. In the early phase, cell wall proteins are synthesized and transported to the periphery. Increased cell wall protein synthesis renders a Golgi-like structure readily visible with electron microscopy (Reiner et al, 1990). The proteins, containing a leucine-rich signature, are redirected to Encystation-specific transport vesicles (ESV), visible under light microscopy (Hehl et al, 2000).
The late phase involves the assembly of the cyst wall and morphological change. The assembly begins with cyst wall filaments, then the filamentous layer of the cell wall (Adam, 2001). During the course of late-phase encystations, the trophozoite loses motility. Eventually a non-adhering cyst enclosing two physically joined trophozoites with four nuclei results.
Pathology
Giardiasis is the clinical manifestation of G. lamblia infection. It is characterized by severe diarrhea, malnutrition weight loss, and slight intestinal epithelial injury. The complete pathogenesis is no well known, but several theories exist.
The disruption of the small intestine epithelium is proposed to be caused by induced apoptosis (Chin et al, 2002). Recently this theory was proved in vitro. Both intrinsic and extrinsic apoptotic pathways on enterocytes were observed. In addition, Bcl-2 was downregulated, and caspase-3 action was assayed (Panaro et al, 2007). However this theory appears to be strain-specific. It has also been shown that G. lamblia increases permeability of single-layered duodenal epithelium in vitro, by phosphorylating F-actin and zonaoccludens in enterocytes (Scott et al, 2002).
In addition, immmune evasion of G. lamblia is achieved through variation in expression of variant-specific surface proteins (VSP), a family of variable antigenic makers of unknown function (Singer et al, 2000). Selective pressure on parasite is both positive and negative, and is dependent on the VSP. Host-specific factors may also involved.
Although the most prominent clinical manifestations of Giardiasis are diarrhea and malabsorption, detailed mechanisms are not completely characterized. However, colonization of the microbe appears cause microvillus shortening (Scott et al, 2000), villous flattening or atrophy (Williamson et al., 2000). It is thought that these mechanisms may work synergistically with other mechanisms such as inbhition of disaccharase and protease to produce the overall symptoms (Muller et al, 2005).
Recently, a study has shown that in Giardiasis, diarreha is a causative combination of leak flux, malabsorption and malsecretion: downregulation of tight junction protein claudin 1, and epithelial apotosis causes failure of sodium-dependent glucose absorption, which results in active chloride ion secretion. Consequently, water enters the lumen, eliciting diarrhea (Troeger, 2007).
Application to Biotechnology
None has been documented to date.
Current Research
G. lamblia is rigorously under research for its significance in epidemiology, clinical medicine and evolutionary biology.
- Due to the widespread of waterborne disease casued by G. lamblia, it is one major field of study for epidemiologists. Poereira et al evaluated the prevalence of Giardiasis among hospitalized children suffering from diarrhea in Goiânia, Brazil and determined several risk factors for G. lamblia infection (Pereira et al, 2007). These risk factors include: number of children in the household, food hygiene, presence of cats and attendance to day-care centers. Similar studies are currently held by the CDC and other government agencies worldwide (CDC, 2004). Identification of such risk factors allow enable policy makers to target the disease in terms of of legislation and public health services.
- Drug resistance of G. lamblia (strain WB C6 clones) against nitazoxanide and metronidazole has been studied (Muller et al, 2007). The transcription of several proteins involved in the resistance formation, such as pyruvate oxireductases 1 and 2, nitroreductase, VSPs, and protein disulfide isomerases 2 and 4, were quantified and correlated with each strain. And it was found that each strain, synthesis of a particular set of proteins was inhibited. Moreover, it was found that VSP expression was altered dramatically by the drugs. The same researchers are planning to combine genomic and proteonomic methods to understand cellular mechanisms in details, and their role in drug resistance in the parasite.
- A study of the genetic structure of G. lamblia (Yee et al, 2007), has located genes for core histones H2a, H2b, H3 and H4 but not the gene for linker histone H1. Sequence comparison shows similar sequence in both coding and 5’ non-coding regions. This is indicative of recent gene-duplication or conversion. This study suggests an alternative mechanism of gene-compacting in G. lamblia, which might be accomplished without the H1 histone, to optimize the transcription of the gene-rich DNA of the species. Such understanding would be fundemental in knowing the gene expression in this species.
- The Giardia lamblia genome project at the Marine Biological Laboratory in Masshuttes, in which shotgun sequencing is used to obtain the full genome of G. lamblia, is currently under way. Annotated genome assembly is released regularly and is made available to researchers worldwide. The analysis of gene function is done currently in collaboration with Dr. Frances Gillin at UCSD molecular pathology. This study is of great significance in evolutionary biology, as it will provide insight into the history of G. lamblia': its relation with the mitochondria, and its role in serving as the bridge between prokaryotes and eukaryotes. The full genome will also assist researchers in identifying possible genes that codes for toxins and possibly derive drugs that could inhibit the parasite.
References
- Adam, RD, & Adam. 2001. Biology of Giardia lamblia. Clinical microbiology reviews, 14(3), 447-.
- Aggarwal, A., J. W. Merritt, Jr., and T. E. Nash. 1989. Cysteine-rich variant surface proteins of Giardia lamblia. Mol. Biochem. Parasitol. 32:39–47.
- Ali, SA, & Hill, DR. 2003. Giardia intestinalis. Current opinion in infectious diseases, 16(5), 453-60.
- Bernal, R. M., R. Tovar, J. I. Santos, and M. L. Munoz. 1998. Possible role of calmodulin in excystation of Giardia lamblia. Parasitol. Res. 84:687–693.
- Brown, D.M., Upcroft, J.A., Upcroft, P. 1995. Free radical detoxification in Giardia duodenalis. Mol Biochem Parasitol 72: 47–56.
- Brown, D.M., Upcroft, J.A., Upcroft, P. 1996b. A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol Biochem Parasitol 83: 211–220.
- Center for Disease Control, Giardia Infection Fact Sheet. 2004. Retrieved 2007 August 29.
- Chin AC, Teoh DA, Scott KG, et al. 2002. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun 2002; 70:3673–3680.
- Corrêa, G, Morgado-Diaz, JA, & Benchimol, M., 2004. Centrin in Giardia lamblia - ultrastructural localization. FEMS microbiology letters, 233(1), 91-6.
- Edwards, M. R., L. A. Knodler, J. R. Wilson, and P. J. Schofield. 1993. The transport and metabolism of alanine by Giardia intestinalis. Mol. Biochem. Parasitol. 61:49–57.
- Edwards, M. R., P. J. Schofield, W. J. O’Sullivan, and M. Costello. 1992. Arginine metabolism during culture of Giardia intestinalis. Mol. Biochem. Parasitol. 53:97–103.
- Elmendorf, HG, Dawson, SC, & McCaffery, JM. 2003. The cytoskeleton of Giardia lamblia. International Journal for Parasitology, 33(1), 3-28.
- Farthing MJG, Pereira MEA, Keusch GT 1986. Description and characterization of a surface lectin from Giardia lamblia. Infect Immunol 1986;51:661-7.
- Feely, D. E., J. V. Schollmeyer, and S. L. Erlandsen. 1982. Giardia spp.: Distribution of contractile proteins in the attachment organelle. Exp. Parasitol. 53:145–154.
- Gillin, F. D., D. S. Reiner, and S. E. Boucher. 1988. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infect. Immun. 56:705–707.
- Gillin, F. D., Reiner, D. S., and McCaffery, J. M. 1996. Cell biology of the primitive eukaryote Giardia lamblia. Annu. Rev. Microbiol. 50, 679-705
- Hashimoto T, Sanchez LB, Shirakura T, Muller M, Hasegawa M. 1998. Secondary absence of mitochondria in Giardia lamblia and Trichomonas vaginalis revealed by valyl-tRNA synthetase phylogeny. Proc Natl Acad Sci USA 1998;95:6860-5.
- Hashimoto, T., L. B. Sanchez, T. Shirakura, M. Muller, and M. Hasegawa. 1998. Secondary absence of mitochondria in Giardia lamblia and Trichomonas vaginalis revealed by valyl-tRNA synthetase phylogeny. Proc. Natl. Acad. Sci. USA 95:6860–6865.
- Hehl, A. B., M. Marti, and P. Kohler. 2000. Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Mol. Biol. Cell 11:1789–1800.
- Holberton DV and Marshall J. 1995. Analysis of consensus sequence patterns in Giardia cytoskeleton gene promoters. Nucleic Acids Res 1995;23:2945-53. Link.
- Holberton DV. 1974. Attachment of Giardia-a hydrodynamic model based on flagellar activity. J Exp Biol 1974;60:207-21.
- Kane, A. V., Ward, H. D., Keusch, G. T., and Pereira, M. E. A. 1991. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J. Parasitol. 77, 974-981
- Ladeira, RB, Freitas, MA, Silva, EF, et al. 2005. Glycogen as a carbohydrate energy reserve in trophozoites of Giardia lamblia. Parasitology research, 96(6), 418-21.
- Le Blancq, S. M., R. S. Kase, and L. H. Van der Ploeg. 1991. Analysis of a Giardia lamblia rRNA encoding telomere with [TAGGG]n as the telomere repeat. Nucleic Acids Res. 19:5790–5790.
- Lei Li, Ching C. Wang 2006. A likely molecular basis of the susceptibility of Giardia lamblia Towards oxygen Molecular Microbiology 59 (1), 202–211.
- Lloyd, D, Harris, JC, Maroulis, S, et al. 2002. The "primitive" microaerophile Giardia intestinalis (syn. lamblia, duodenalis) has specialized membranes with electron transport and membrane-potential-generating functions. Microbiology, 148(Pt 5), 1349-54.
- Lujan, H. D., A. Marotta, M. R. Mowatt, N. Sciaky, J. Lippincott-Schwartz, and T. E. Nash. 1995. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 270: 4612–4618.
- Meng, T. C., M. L. Hetsko, and F. D. Gillin. 1996. Inhibition of Giardia lamblia excystation by antibodies against cyst walls and by wheat germ agglutinin. Infect. Immun. 64:2151–2157.
- Michels, PA, & Rigden, DJ. 2006. Evolutionary analysis of fructose 2,6-bisphosphate metabolism. IUBMB life, 58(3), 133-41.
- Muller N, Allmen NV. 2005. Recent insights into the mucosal reactions associated with Giardia lamblia infections. International Journal for Parasitology 35 (2005) 1339–1347
- Muller, J, & Muller. 2007. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. The journal of antimicrobial chemotherapy, 60(2), 280-.
- Nygaard, T., C. C. Bennett, G. Grossman, M. R. Edwards, and P. J. Schofield. 1994. Efflux of alanine by Giardia intestinalis. Mol. Biochem. Parasitol. 64:145–152.
- Paget, T. A., E. L. Jarroll, P. Manning, D. G. Lindmark, and D. Lloyd. 1989. Respiration in the cysts and trophozoites of Giardia muris. J. Gen. Microbiol. 135:145–154.
- Panaro MA, Cianciulli A, Mitolo V, Mitolo CI, Acquafredda A, Brandonisio O, Cavallo P. 2007. Caspase-dependent apoptosis of the HCT-8 epithelial cell line induced by the parasite Giardia intestinalis. FEMS Immunol Med Microbiol. 2007 Aug 20;
- Papanastasiou, P., T. Bruderer, Y. Li, C. Bommeli, and P. Kohler. 1997. Primary structure and biochemical properties of a variant-specific surface protein of Giardia. Mol. Biochem. Parasitol. 86:13–27.
- Pereira, MGC, ATWILL, ER and BARBOSA, AP. 2007. Prevalence and associated risk factors for Giardia lamblia infection among children hospitalized for diarrhea in Goiânia, Goiás State, Brazil. Rev. Inst. Med. trop. S. Paulo, May/June 2007, vol.49, no.3, p.139-145
- Reiner, D. S., M. McCaffery, and F. D. Gillin. 1990. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur. J. Cell Biol. 53:142–153.
- Roger AJ, Svard SG, Tovar J et al. 1998. A mitochondrial-like chaperonin 60 gene in Giardia lamblia: evidence that diplomonads once harbored an endosymbiont related to the progenitor of mitochondria. 1998. Proc Natl Acad Sci USA;95:229-34.
- Sanchez, L.B., Elmendorf, H., Nash, T.E., Muller, M. 2001. NAD(P)H:menadione oxidoreductase of the amitochondriate eukaryote Giardia lamblia: a simpler homologue of the vertebrate enzyme. Microbiology 147: 561–570.
- Scott KG, Meddings JB, Kirk DR, et al. 2002. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 123:1179–1190.
- Scott, K.G., Logan, M.R., Klammer, G.M., Teoh, D.A., Buret, A.G., 2000. Jejunal brush border microvillous alterations in Giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infect. Immun. 68, 3412–3418.
- Singer SM, Elmendorf HG, Conrad JT, Nash TE. 2000. Biological Selection of Variant-Specific Surface Proteins in Giardia lamblia. J. Infectious Diseases 2001;183:119-124
- Soltys, B. J., M. Falah, and R. S. Gupta. 1996. Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to Bip. J. Cell Sci. 109:1909–1917.
- Teodorovic, S. Walls, CD. and Elmendorf, HG. 2007. Bidirectional transcription is an inherent feature of Giardia lamblia promoters and contributes to an abundance of sterile antisense transcripts throughout the genome. Nucleic Acids Research, 2007, Vol. 35, No. 8 2544-2553. Link.
- Thompson, R.C.A., 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30, pp. 1259–1267.
- Troeger, H, Epple, HJ, Schneider, T, et al., 2007. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut, 56(3), 328-35.
- Yee, J, Tang, A, Lau, WL, et al. 2007. Core histone genes of Giardia intestinalis: genomic organization, promoter structure, and expression. BMC molecular biology, 8, 26-.
- Yu, LZ. Birky, C. Willia, Jr, Adam, RD. 2002. The Two Nuclei of Giardia Each Have Complete Copies of the Genome and Are Partitioned Equationally at Cytokinesis. EUKARYOTIC CELL, Apr. 2002, p. 191–199 a
Edited by student D.Lau of Rachel Larsen
Edited by KLB