Gluconobacter morbifer G707: Difference between revisions
Mary.cameron (talk | contribs) |
Mary.cameron (talk | contribs) |
||
| (43 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
[[File:Tree_of_Life.jpeg|right|400px|The three domains of life, with phylum Proteobacteria. ''From Carl Zimmer's blog, The Loom.'']] | |||
==Classification== | ==Classification== | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
| Line 10: | Line 12: | ||
''Gluconobacter morbifer G707'' [1] | ''Gluconobacter morbifer G707'' [1] | ||
[http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&id=1088869&lvl=3&lin=f&keep=1&srchmode=1&unlock NCBI:] | |||
==Description and significance== | ==Description and significance== | ||
''Gluconobacter morbifer'' G707 is a non-motile, aerobic, rod-shaped bacteria found in Seoul, South Korea [2]. Isolated from the gastrointestinal tract of the fruit fly ''Drosophila melanogaster'', this bacteria is described as being a commensal symbiont [2]; however, research on the fruit fly’s gut commensal community provides evidence that an abundance of ''G. morbifer'' causes apoptosis of gut cells and an early death to its host [3]. In immune-deregulated hosts, ''G. morbifer'' is associated with the unique trait of pathogenesis promotion, making this bacteria’s genome sequence particularly valuable in furthering scientific understanding of bacterial pathogenesis in varying host environs [4]. | ''Gluconobacter morbifer'' G707 is a non-motile, aerobic, rod-shaped bacteria found in Seoul, South Korea [2]. Isolated from the gastrointestinal tract of the fruit fly ''Drosophila melanogaster'', this bacteria is described as being a commensal symbiont [2]; however, research on the fruit fly’s gut commensal community provides evidence that an abundance of ''G. morbifer'' causes apoptosis--the process of programmed cell death--of gut cells and an early death to its host [3]. In immune-deregulated hosts, ''G. morbifer'' is associated with the unique trait of pathogenesis promotion, making this bacteria’s genome sequence particularly valuable in furthering scientific understanding of bacterial pathogenesis in varying host environs [4]. | ||
==Genome structure== | ==Genome structure== | ||
The ''G. morbifer'' G707 genome contains 2,899 genes, 50 | The ''G. morbifer'' G707 genome contains 2,887,061 DNA base pairs and 2,899 genes [2]. Of those genes, 2,849 are protein coding genes, with 50 labeled as RNA genes [2]. G+C genome content is 59.04% over 19 DNA scaffolds, all of which are linear [2]. Approximately 56% of protein coding genes possess a predicted function [2]. ''G. morbifer'' exhibits a high similarity of 16 rRNA gene sequence to ''Gluconobacter albidus'' BCC 14434 (97.9%), ''Gluconobacter cerinus'' ATCC 19441 (97.6%), ''Gluconobacter oxydans'' ATCC 19357 (97.5%), ''Gluconobacter frateurii'' ATCC 49207 (97.4%), and ''Gluconobacter thailandicus'' F149-1 (97.4%) [3]. | ||
==Cell and colony structure== | ==Cell and colony structure== | ||
''G. morbifer'' are nonsporulating, rod-shaped bacteria measuring 0.7 by 1.8 to 2.0 | ''G. morbifer'' are nonsporulating, rod-shaped bacteria measuring 0.7 by 1.8 to 2.0 µm, with circular, pink colonies measuring 1.0 to 1.5 mm in diameter (at 25 degree C and aerobic conditions maintained over the course of a two day period on mannitol-agar) [3]. Some cells stain Gram positive while others stain Gram negative, making this a variable Gram staining bacteria [3]. | ||
==Metabolism== | ==Metabolism== | ||
''G. morbifer'' exhibits aerobic respiration using an Ubiquinol oxidation pathway with oxygen and proton transport [2]. ''G. morbifer'' is generally auxotrophic, meaning it is unable to manifest many of its own nutritional requirements—like | ''G. morbifer'' exhibits aerobic respiration using an Ubiquinol oxidation pathway with oxygen and proton transport [2]. ''G. morbifer'' is generally auxotrophic, meaning it is unable to manifest many of its own nutritional requirements—like metabolites and amino acids—for growth, making it a fastidious microbe; however, it is prototrophic for L-aspartate and L-glutamate [2]. This bacteria also is characterized as having an incomplete Coenzyme A biosynthesis [2]. | ||
==Ecology== | ==Ecology== | ||
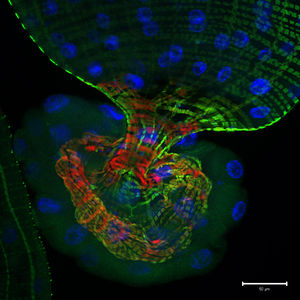
G707, originally isolated in Seoul, South Korea, exhibit endosymbiotic extracellular physical interactions, living commensally within the intestinal microflora of the fruit fly ''Drosophila melanogaster'' [2]. Despite being described as commensal, the symbiotic relationship between ''G. morbifer'' and | [[File:Fruit_Fly_Gut.jpeg|left|300px|50 µm. A photomicrographic optical section through the tip of a D. melanogaster larval gut. The green shows activity of the Notch signaling pathway; the blue and red represent stained nuclear and cytoskeletal markers respectively. The image was captured by Jessica Von Stetina of Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, USA, and shared by www.nikonsmallworld.com.]] ''G. morbifer'' G707, originally isolated in Seoul, South Korea, exhibit endosymbiotic extracellular physical interactions, living commensally within the intestinal microflora of the fruit fly ''Drosophila melanogaster'' [2]. Despite being described as commensal, the symbiotic relationship between ''G. morbifer'' and host is ultimately dependent upon the condition of the host’s immunity; the strain will display dominance when the host’s immune homeostasis is compromised, resulting in pathology and gradual host opportunistic infection [4]. 3.6 to 7.8 is the recognized optimal pH range for growth [3]. The bacteria has been successfully cultured on mannitol-agar plates (up to 3% NaCl) incubated at 25°C, and has also been observed growing on conventional media (e.g., TSA, R2A) with optimal mesophilic growth ranging 25-30°C [3]. ''G. morbifer'' is oxidase negative and catalase positive [3]. | ||
==Pathology== | ==Pathology== | ||
When inhabiting a ''Drosophila melanogaster'' host, | When inhabiting a ''Drosophila melanogaster'' host, mesophilic ''G. morbifer'' is normally harmless, living commensally with other ''Drosophila'' intestinal microbiota [4]. However, when the host’s gut immunity experiences overactivation, ''G. morbifer'' becomes dominant, causing apoptosis (programmed cell death) and early termination of the host [4]. Virulence factors associated with ''G. morbifer's'' promotion of colonization within its host (and thus, gut pathology) is currently unknown [4]. With G707’s unique characteristic of becoming pathogenic in an immune-compromised host, its genome sequence possesses potentially substantial value in terms of improving scientific understanding of pathogenesis in varying host environs at the bacterial level [4]. While official results have yet to be published, it is assumed that in further exploration of ''G. morbifer’s'' role in fruit fly ecology that a relationship between ''D. melanogaster'' physiology and its genotype-based commensal gut community of bacterial strains—specifically with ''G. morbifer''—will be confirmed [3]. | ||
==References== | ==References== | ||
Latest revision as of 01:15, 2 May 2013
A Microbial Biorealm page on the genus Gluconobacter morbifer G707
Classification
Higher order taxa
Bacteria; Proteobacteria; Alphaproteobacteria; Rhodospirillales; Acetobacteraceae; Gluconobacter [1]
Species
Gluconobacter morbifer G707 [1] NCBI:
Description and significance
Gluconobacter morbifer G707 is a non-motile, aerobic, rod-shaped bacteria found in Seoul, South Korea [2]. Isolated from the gastrointestinal tract of the fruit fly Drosophila melanogaster, this bacteria is described as being a commensal symbiont [2]; however, research on the fruit fly’s gut commensal community provides evidence that an abundance of G. morbifer causes apoptosis--the process of programmed cell death--of gut cells and an early death to its host [3]. In immune-deregulated hosts, G. morbifer is associated with the unique trait of pathogenesis promotion, making this bacteria’s genome sequence particularly valuable in furthering scientific understanding of bacterial pathogenesis in varying host environs [4].
Genome structure
The G. morbifer G707 genome contains 2,887,061 DNA base pairs and 2,899 genes [2]. Of those genes, 2,849 are protein coding genes, with 50 labeled as RNA genes [2]. G+C genome content is 59.04% over 19 DNA scaffolds, all of which are linear [2]. Approximately 56% of protein coding genes possess a predicted function [2]. G. morbifer exhibits a high similarity of 16 rRNA gene sequence to Gluconobacter albidus BCC 14434 (97.9%), Gluconobacter cerinus ATCC 19441 (97.6%), Gluconobacter oxydans ATCC 19357 (97.5%), Gluconobacter frateurii ATCC 49207 (97.4%), and Gluconobacter thailandicus F149-1 (97.4%) [3].
Cell and colony structure
G. morbifer are nonsporulating, rod-shaped bacteria measuring 0.7 by 1.8 to 2.0 µm, with circular, pink colonies measuring 1.0 to 1.5 mm in diameter (at 25 degree C and aerobic conditions maintained over the course of a two day period on mannitol-agar) [3]. Some cells stain Gram positive while others stain Gram negative, making this a variable Gram staining bacteria [3].
Metabolism
G. morbifer exhibits aerobic respiration using an Ubiquinol oxidation pathway with oxygen and proton transport [2]. G. morbifer is generally auxotrophic, meaning it is unable to manifest many of its own nutritional requirements—like metabolites and amino acids—for growth, making it a fastidious microbe; however, it is prototrophic for L-aspartate and L-glutamate [2]. This bacteria also is characterized as having an incomplete Coenzyme A biosynthesis [2].
Ecology
G. morbifer G707, originally isolated in Seoul, South Korea, exhibit endosymbiotic extracellular physical interactions, living commensally within the intestinal microflora of the fruit fly Drosophila melanogaster [2]. Despite being described as commensal, the symbiotic relationship between G. morbifer and host is ultimately dependent upon the condition of the host’s immunity; the strain will display dominance when the host’s immune homeostasis is compromised, resulting in pathology and gradual host opportunistic infection [4]. 3.6 to 7.8 is the recognized optimal pH range for growth [3]. The bacteria has been successfully cultured on mannitol-agar plates (up to 3% NaCl) incubated at 25°C, and has also been observed growing on conventional media (e.g., TSA, R2A) with optimal mesophilic growth ranging 25-30°C [3]. G. morbifer is oxidase negative and catalase positive [3].
Pathology
When inhabiting a Drosophila melanogaster host, mesophilic G. morbifer is normally harmless, living commensally with other Drosophila intestinal microbiota [4]. However, when the host’s gut immunity experiences overactivation, G. morbifer becomes dominant, causing apoptosis (programmed cell death) and early termination of the host [4]. Virulence factors associated with G. morbifer's promotion of colonization within its host (and thus, gut pathology) is currently unknown [4]. With G707’s unique characteristic of becoming pathogenic in an immune-compromised host, its genome sequence possesses potentially substantial value in terms of improving scientific understanding of pathogenesis in varying host environs at the bacterial level [4]. While official results have yet to be published, it is assumed that in further exploration of G. morbifer’s role in fruit fly ecology that a relationship between D. melanogaster physiology and its genotype-based commensal gut community of bacterial strains—specifically with G. morbifer—will be confirmed [3].
References
[1] http://www.ncbi.nlm.nih.gov/bioproject/73361; NCBI
[2] http://img.jgi.doe.gov/cgi-bin/w/main.cgi?section=TaxonDetail&page=taxon Detail&taxon_oid=2513237393; Markowitz1, V., Chen, I., Palaniappan, K., Chu, K., Szeto, E., Grechkin, Y., Ratner, A., Jacob, B., Huang, J., Williams, P., Huntermann, M., Anderson, I., Mavromatis, K., Ivanova, N., Kyrpides, N. 2012. IMG: the integrated microbial genomes database and comparative analysis system. [Accessed 22 January 2013].
[3] Roh, S., Nam, Y., Chang, H., Kim, K., Kim, M., Ryu, J., Kim, S., Lee, W., Bee, J. 2008. Phylogenic Characterization of Two Novel Commensal Bacteria Involved with Innate Immune Homeostasis in Drosophila melanogaster. Appl Environ Microbio. 74(20): 6171-6177.
[4] Kim, E., Kim, S., Nam, H., Myoung, K., Lee. K., Choi, S., Seo, Y., You, H., Kim, B., Lee, W. 2012. Draft Genome Sequence of Gluconobacter morbifer G707, a Pathogenic Gut Bacterium Isolated from Drosophila melanogaster Intestine. J Bacteriol. 194(5): 1245. Doi: 10.1128/JB.06670-11.
Edited by Lon R. Cameron, student of Dr. Lisa R. Moore, University of Southern Maine, Department of Biological Sciences, http://www.usm.maine.edu/bio