Gut Microbiota and Autism: Difference between revisions
| (72 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
<br>By Bailey Fitzgerald <br> | <br>By Bailey Fitzgerald <br> | ||
<br> | <br> | ||
[[Image:Gutbrain.png|thumb|300px|right|<b>Figure 1:</b> Diagram of the gut-brain axis and its influence in autism spectrum disorder. Photo credit: Chidambaram et al., https://link.springer.com/chapter/10.1007/978-3-030-30402-7_21.]] | [[Image:Gutbrain.png|thumb|300px|right|<b>Figure 1:</b> Diagram of the gut-brain axis and its influence in autism spectrum disorder. Photo credit: Chidambaram et al., https://link.springer.com/chapter/10.1007/978-3-030-30402-7_21.]] | ||
A healthy adult human intestine harbors billions of bacteria of over 1000 different species.<ref name=Maukonen>Maukonen J, Saarela M. Human gut microbiota: does diet matter? Proceedings of the Nutrition Society. 2015 Feb;74(1):23–36.</ref><ref name=Bull>Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas). 2014 Dec;13(6):17–22.</ref> These bacteria play a large role in human health through digestion and aiding in immune system development.<ref name=Bull/> However, these bacteria can also have a direct influence on their human host’s behavior. Gut microbes have the ability to bidirectionally communicate with the central nervous system and influence both emotional and cognitive centers of the brain.<ref name=Carabotti>Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.</ref> This makes maintenance of the gut microbiome especially important in people with a variety of neurological conditions, including autism spectrum disorder (ASD). | A healthy adult human intestine harbors billions of bacteria of over 1000 different species.<ref name=Maukonen>Maukonen J, Saarela M. Human gut microbiota: does diet matter? Proceedings of the Nutrition Society. 2015 Feb;74(1):23–36.</ref><ref name=Bull>Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas). 2014 Dec;13(6):17–22.</ref> These bacteria play a large role in human health through digestion and aiding in immune system development.<ref name=Bull/> However, these bacteria can also have a direct influence on their human host’s behavior. Gut microbes have the ability to bidirectionally communicate with the central nervous system and influence both emotional and cognitive centers of the brain.<ref name=Carabotti>Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.</ref> This makes maintenance of the gut microbiome especially important in people with a variety of neurological conditions, including autism spectrum disorder (ASD). | ||
<br><br> | <br><br> | ||
Autism spectrum disorder is a neurological and developmental disorder characterized by deficits in social communication and social interaction, repetitive patterns of behavior or activities, and restricted interests.<ref name=DSM>American Psychiatric Association, American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013.</ref> ASD can range in severity from very high-functioning (formerly known as Asperger’s syndrome) in which the individual is able to go about daily life with little to no assistance to low-functioning individuals that need assistance with basic care and needs or anywhere in between. It is also common to have other diseases and conditions along with autism spectrum disorder. These comorbidities can include sleep disorders, seizure disorders, gastrointestinal disorders, metabolic disorders, and hormonal dysfunction.<ref name=Bauman>Bauman ML. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics. 2010 Jul;7(3):320–7.</ref> | Autism spectrum disorder is a neurological and developmental disorder characterized by deficits in social communication and social interaction, repetitive patterns of behavior or activities, and restricted interests.<ref name=DSM>American Psychiatric Association, American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013.</ref> The CDC estimates that approximately 1 in every 54 children in the United States is affected by autism spectrum disorder.<ref name=CDC>CDC. Autism and Developmental Disabilities Monitoring (ADDM) Network | CDC [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2021 Apr 7]. Available from: https://www.cdc.gov/ncbddd/autism/addm.html</ref> ASD can range in severity from very high-functioning (formerly known as Asperger’s syndrome) in which the individual is able to go about daily life with little to no assistance to low-functioning individuals that need assistance with basic care and needs, or anywhere in between. It is also common to have other diseases and conditions along with autism spectrum disorder. These comorbidities can include sleep disorders, seizure disorders, gastrointestinal disorders, metabolic disorders, and hormonal dysfunction.<ref name=Bauman>Bauman ML. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics. 2010 Jul;7(3):320–7.</ref> | ||
<br><br> | <br><br> | ||
Gastrointestinal (GI) issues, such as frequent abdominal pain, gaseousness, diarrhea, constipation or pain on stooling, are a very common comorbidity in ASD patients.<ref name=Li>Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci [Internet]. 2017 [cited 2021 Mar 17];11. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2017.00120/full?fbclid=IwAR04gQH8jD4LVLXEJnl7MfXsCyDR7USEauiGGIc1BXJY48GTn2L5GQg-Hk0</ref><ref name=Chaidez/> Studies have found that 23 to 70% of people with ASD suffer from gastrointestinal dysfunction, with the wide range attributed to variances in survey methods.<ref name=Chaidez>Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J Autism Dev Disord. 2014 May 1;44(5):1117–27.</ref> Additionally, the prevalence of more gastrointestinal symptoms was strongly correlated with the severity of autism in patients.<ref name=Adams>Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterology. 2011 Mar 16;11(1):22.</ref> These gastrointestinal issues can be attributed to a disturbance | Gastrointestinal (GI) issues, such as frequent abdominal pain, gaseousness, diarrhea, constipation or pain on stooling, are a very common comorbidity in ASD patients.<ref name=Li>Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci [Internet]. 2017 [cited 2021 Mar 17];11. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2017.00120/full?fbclid=IwAR04gQH8jD4LVLXEJnl7MfXsCyDR7USEauiGGIc1BXJY48GTn2L5GQg-Hk0</ref><ref name=Chaidez/> Studies have found that 23% to 70% of people with ASD suffer from gastrointestinal dysfunction, with the wide range attributed to variances in survey methods.<ref name=Chaidez>Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J Autism Dev Disord. 2014 May 1;44(5):1117–27.</ref> Additionally, the prevalence of more gastrointestinal symptoms was strongly correlated with the severity of autism in patients.<ref name=Adams>Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterology. 2011 Mar 16;11(1):22.</ref> These gastrointestinal issues can be attributed to a disturbance in normal gut flora composition.<ref name=Adams/> Although the cause of the abnormal gut microbiome in patients with ASD is not certain, researchers hypothesize that it could be due to atypical eating habits.<ref name=Mulle>Mulle JG, Sharp WG, Cubells JF. The Gut Microbiome: A New Frontier in Autism Research. Curr Psychiatry Rep. 2013 Feb 1;15(2):1–9.</ref> Thus, therapies targeting the gut microbiome could have potential in treating and managing autism spectrum disorders. Because the exact causes of ASD and the mechanisms behind the disorder are difficult to identify, there are very few effective therapies available.<ref name=Rossignol>Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Molecular Psychiatry. 2012 Apr;17(4):389–401.</ref> This makes the potential of microbiome treatments for ASD even more promising and hopeful for managing the disorder. | ||
==Composition of the ASD Microbiome== | |||
[[Image:ASDbacteria.png|thumb|300px|right|<b>Figure 2:</b> Composition of gut microbiome in healthy children (HC), children with developmental disorders (PDD-NOS), and children with ASD (AD). Red signifies more prevalent bacterial species while green denotes less prevalence. Samples are grouped using double dendrogram representation (left side) to identify similarities in composition. Photo credit: De Angelis et al., https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0076993#pone-0076993-g003.]] | |||
== | The microbiome of individuals with autism spectrum disorder differs from that of the average person. Firstly, the intestinal environment itself is abnormal in patients with ASD compared to the typical person. Approximately one third of patients with ASD displayed a higher percentage of abnormal permeability in their intestines compared to the approximately 5% of abnormal permeability in the control population.<ref name=deMagistris>de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the Intestinal Barrier in Patients With Autism Spectrum Disorders and in Their First-degree Relatives. Journal of Pediatric Gastroenterology and Nutrition. 2010 Oct;51(4):418–24.</ref> This increased intestinal permeability causes an increased antigenic load from the GI tract. This allows more chemical signals originating from the GI tract to enter the circulation and then cross over the blood-brain barrier to deliver these chemical signals to the brain where they induce immune responses.<ref name=Li/> Therefore, the microbiota of ASD patients may have even more influence through the gut-brain axis than in the average person. | ||
<br><br> | |||
Patients with ASD have different species of bacteria prevalent in their gut microbiome. The maternal immune activation (MIA) mouse model is an ideal model for studying ASD as it displays many features of the disorder. One study found that offspring of mothers with MIA had more <i>Porphyromonadaceae, Prevotellaceae, </i>unclassified<i> Bacteroidales, </i>and<i> Lachnospiraceae</i> bacterias, whereas control offspring had more <i>Ruminococcaceae, Erysipelotrichaceae, </i>and <i>Alcaligenaceae</i> bacterias.<ref name=Hsiao>Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013 Dec 19;155(7):1451–63.</ref> Another model system for studying ASD can be created by inducing autistic-like behaviors in offspring of mice through the administration of valproic acid in pregnant mice. In all offspring of the VPA treated mice, <i>Bacteroidetes</i> and <i>Desulfovibrionales</i> increased in prevalence while <i>Firmicutes</i> decreased. Additionally, male VPA offspring possessed more <i>Alistipes, Enterorhabdus, Mollicutes</i> and <i>Erysipelotrichalis</i> bacteria compared to their female siblings.<ref name=deTheige>de Theije CGM, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain, Behavior, and Immunity. 2014 Mar 1;37:197–206.</ref> | |||
<br><br> | <br><br> | ||
Analyses of the microbiota within humans with ASD have been mostly consistent with the findings of these animal models. The microbiome of children with autism spectrum disorder has been shown to be less diverse overall and contains lower levels of <i>Bifidobacterium</i> and <i>Firmicutes</i> while the prevalence of <i>Lactobacillus, Clostridium, Bacteroidetes, Desulfovibrio, Caloramator,</i> and <i>Sarcina</i> bacterias are higher.<ref name=Finegold02>Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E, et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clinical Infectious Diseases. 2002 Sep 1;35(Supplement_1):S6–16.</ref><ref name=Finegold10>Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010 Aug 1;16(4):444–53.</ref><ref name=Adams/><ref name=Finegold11>1. Finegold SM. Desulfovibrio species are potentially important in regressive autism. Medical Hypotheses. 2011 Aug 1;77(2):270–4.</ref><ref name=DeAngelis>De Angelis M, Piccolo M, Vannini L, Siragusa S, Giacomo AD, Serrazzanetti DI, et al. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLOS ONE. 2013 Oct 9;8(10):e76993.</ref><ref name=Li/> Autistic children specifically with GI symptoms had lower prevalence of <i>Prevotella, Coprococcus, </i>and unclassified <i>Veillonellaceae</i> bacteria. Given that these bacteria are versatile carbohydrate-degrading and/or fermenting bacteria, it would suggest that these differences could be attributed to atypical diet and feeding behaviors associated with ASD; however, the study showed no correlation between diet patterns and microbial diversity or abundance.<ref name=Kang>Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLOS ONE. 2013 Jul 3;8(7):e68322.</ref> | |||
<br><br> | |||
The increased abundance of <i>Clostridium</i> bacteria in the gut microbiome of individuals with ASD is cause for concern. <i>Clostridium</i> bacteria are known to be toxin-producers, their products of which include neurotoxins that could be harmful to individuals with ASD through the gut-brain axis.<ref name=Hatheway>Hatheway CL. Toxigenic clostridia. Clinical Microbiology Reviews. 1990 Jan 1;3(1):66–98.</ref> Additionally, this bacteria may contribute to gut dysfunction and GI symptoms.<ref name=Parracho>Parracho HM, Bingham MO, Gibson GR, McCartney A. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of Medical Microbiology. 2005;54(10):987–91.</ref> Given that increased GI symptoms and ASD severity show positive correlation, <i>Clostridium</i> bacteria may be associated with the development of certain autistic characteristics.<ref name=Adams/><ref name=Parracho/> | |||
==Development of Gut Microbiota and ASD== | ==Development of the Gut Microbiota and ASD== | ||
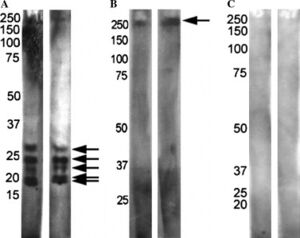
[[Image:antiASD.jpg|thumb|300px|right|<b>Figure 3:</b> Antibodies from mothers of children with autism and mothers of neurotypical children reacted with rat prenatal (PN18) brain proteins on 15% SDS–PAGE. Mothers of autistic children and | [[Image:antiASD.jpg|thumb|300px|right|<b>Figure 3:</b> Antibodies from mothers of children with autism and mothers of neurotypical children reacted with rat prenatal (PN18) brain proteins on 15% SDS–PAGE. Mothers of autistic children and autistic children displayed patterns (A) or (B). Mothers of neurotypical children, neurotypical children, and siblings of autistic children displayed pattern (C). Photo credit: Zimmerman et al., https://www.sciencedirect.com/science/article/pii/S0889159106002947?casa_token=4Of3Dg72tJkAAAAA:xYOYLuKkgkFaK7qXmq4kmazS3iXDrILtzAbjIYNzvxromDMpqUNFWWnBpnXxexqyA9Fuq_omS10#bib24.]] | ||
Autism spectrum disorder has been observed to have both genetic and environmental factors to its development.<ref name=Hallmayer>Hallmayer J. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011 Nov 1;68(11):1095.</ref> Environmental factors specifically affecting the composition and development of the gut microbiome may have an effect on the development of autism spectrum disorder. The most crucial environment in ASD development is the prenatal conditions within their mother’s womb. This is largely determined by maternal health. Several studies have found a significant connection between maternal obesity, gestational diabetes (GDM), and pregestational diabetes (PGDM) in increased risk of ASD.<ref name=Connolly>Connolly N, Anixt J, Manning P, Lin DP-I, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Research. 2016;9(8):829–37.</ref><ref name=Jo>Jo H, Eckel SP, Chen J-C, Cockburn M, Martinez MP, Chow T, et al. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environment International. 2019 Dec 1;133:105110.</ref><ref name=LiM>Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics [Internet]. 2016 Feb 1 [cited 2021 Apr 6];137(2). Available from: https://pediatrics.aappublications.org/content/137/2/e20152206</ref> Having just one of these conditions during pregnancy is associated with a 1.5 to 2 times increased risk factor for ASD. Whereas when obesity and GDM or obesity and PGDM were both present in mothers, ASD risk showed approximately 2 to 4 times increase.<ref name=Connolly/><ref name=LiM/> | Autism spectrum disorder has been observed to have both genetic and environmental factors to its development.<ref name=Hallmayer>Hallmayer J. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011 Nov 1;68(11):1095.</ref> Environmental factors specifically affecting the composition and development of the gut microbiome may have an effect on the development of autism spectrum disorder. The most crucial environment in ASD development is the prenatal conditions within their mother’s womb. This is largely determined by maternal health. | ||
<br><br> | |||
Several studies have found a significant connection between maternal obesity, gestational diabetes (GDM), and pregestational diabetes (PGDM) in increased risk of ASD.<ref name=Connolly>Connolly N, Anixt J, Manning P, Lin DP-I, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Research. 2016;9(8):829–37.</ref><ref name=Jo>Jo H, Eckel SP, Chen J-C, Cockburn M, Martinez MP, Chow T, et al. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environment International. 2019 Dec 1;133:105110.</ref><ref name=LiM>Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics [Internet]. 2016 Feb 1 [cited 2021 Apr 6];137(2). Available from: https://pediatrics.aappublications.org/content/137/2/e20152206</ref> Having just one of these conditions during pregnancy is associated with a 1.5 to 2 times increased risk factor for ASD. Whereas when obesity and GDM or obesity and PGDM were both present in mothers, ASD risk showed approximately 2 to 4 times increase.<ref name=Connolly/><ref name=LiM/> | |||
<br><br> | |||
The maternal immune system and responses can also play a factor in ASD development. Mothers of children with ASD have been shown to possess different antibodies than mothers of neurotypical children.<ref name=Zimmerman>Zimmerman AW, Connors SL, Matteson KJ, Lee L-C, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity. 2007 Mar 1;21(3):351–7.</ref> When reacted with rat prenatal (PN18) brain proteins, mothers of children with ASD and children with ASD showed one of two patterns while control mothers and their neurotypical children typically showed no reactivity (Figure 3). This suggests these antibodies are pathogenic to fetal brains in autism.<ref name=Zimmerman/> Maternal antibodies have also been shown to react with lymphocytes in children with ASD which further suggests reactivity with other tissues in the developing embryo.<ref name=Warren>Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, et al. Detection of Maternal Antibodies in Infantile Autism. Journal of the American Academy of Child & Adolescent Psychiatry. 1990 Nov 1;29(6):873–7.</ref> | |||
<br><br> | |||
A mother’s own microbiota can play a crucial role in fetal development. If the microbiome becomes imbalanced and pathogenic microbes are able to dominate, it can mean more than just illness for an expecting mother. Viral infections during the first trimester and bacterial infections during the second trimester have been associated with greater risk of ASD.<ref name=Altadottir>Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2010 Dec;40(12):1423–30.</ref> Additionally, delivery method can shape the microbiome of newborn babies. Infants delivered vaginally possess gut bacteria very similar to their mother’s vaginal microbiota, mostly composed of <i>Lactobacillus, Prevotella, </i>and<i> Sneathis</i> spp.; infants delivered via Cesarean section have a gut microbiome similar to the composition of their mother’s skin microbiota, mainly consisting of <i>Staphylococcus, Corynebacterium, </i>and<i> Propionibacterium</i> spp.<ref name=Dominguez>Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences. 2010 Jun 29;107(26):11971–5.</ref> Autism spectrum disorder risk for infants delivered through Cesarean section has been found to be higher than in infants delivered vaginally.<ref name=Curran>Curran EA, O’Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, et al. Research Review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Journal of Child Psychology and Psychiatry. 2015;56(5):500–8.</ref> Additionally, antibiotics can restructure the composition of the gut microbiome. In children with ASD, the use of antibiotics is much more common than in their neurotypical counterparts.<ref name=Li/> This leads to further imbalance to their already abnormal microbiome composition. | |||
<br><br> | |||
After birth, diet plays a critical role in shaping and maintaining the human gut microbiome. Risk of ASD has been observed to increase for infants that are not breastfed and decreases with longer periods of breastfeeding.<ref name=Farsi>Al-Farsi YM, Al-Sharbati MM, Waly MI, Al-Farsi OA, Al-Shafaee MA, Al-Khaduri MM, et al. Effect of suboptimal breast-feeding on occurrence of autism: A case–control study. Nutrition. 2012 Jul 1;28(7):e27–32.</ref> However, complications in breastfeeding infants with ASD, such as difficulty with the child’s engagement in the feeding process and temperamental or behavioral issues associated with ASD, may also be to blame for this correlation.<ref name=Mulle>Mulle JG, Sharp WG, Cubells JF. The Gut Microbiome: A New Frontier in Autism Research. Curr Psychiatry Rep. 2013 Feb 1;15(2):1–9.</ref> Later in life, feeding issues associated with ASD may contribute to an imbalanced gut microbiome. 90% of children with ASD display feeding related concerns, the most common of which is food selectivity.<ref name=Ledford>Ledford JR, Gast DL. Feeding Problems in Children With Autism Spectrum Disorders: A Review. Focus Autism Other Dev Disabl. 2006 Aug 1;21(3):153–66.</ref><ref name=Mulle/> This selectivity and imbalanced diet leads to many health concerns such as nutritional deficiencies and GI conditions.<ref name=Mulle/> | |||
<br><br> | <br><br> | ||
== | ==Microbiome Treatments for ASD== | ||
===Antibiotics=== | |||
<br> | [[Image:anticomp.jpg|thumb|300px|right|<b>Figure 4:</b> Composition of gut microbiome before and after antibiotic treatment. Photo credit: Desbonnet et al., https://www.sciencedirect.com/science/article/pii/S0889159115000896?casa_token=z1d3gJ8w0o0AAAAA:dzn_H1ukAq1x05vj2vzH971X9amM4LSbmp3OHg33E7W5elRfah_Cpo_rMk_b9y6VVnCGwEUKrW0.]] | ||
Antibiotics may be a viable option for treating neurological conditions, like autism spectrum disorder. The use of antibiotic treatment in mice was shown to have impacts on anxiety and cognitive behaviors through the alteration of gut-brain communication neuromodulators.<ref name=Desbonnet>Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015 Aug;48:165–73.</ref> Desbonnet et al. administered treatment that restructured the gut microbiome and reduced the richness of bacterial species (Figure 4). This restructured microbiome resulted in altered dynamics of the tryptophan metabolic pathway, and significantly reduced BDNF, oxytocin and vasopressin expression in the brain; changes in these neurochemical levels showed association with reduction of anxiety, non-spatial memory, and performance in the social transmission of food preference.<ref name=Desbonnet/> Overall, this study shows evidence of interactions between antibiotic treatments and neurological processes, solidifying antibiotics as a possible therapy for neurological disorders. | |||
<br><br> | |||
Antibiotic treatment has been shown to be beneficial in treating ASD patients that also suffer from GI symptoms. After a short course of oral vancomycin treatment, children with ASD exhibited both reduced diarrhea and improved behavior and communication.<ref name=Sandler>Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen M-L, et al. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J Child Neurol. 2000 Jul 1;15(7):429–35.</ref> These results suggest the treatment caused a reduction of pathogenic bacteria in the gut microbiome and provides further evidence of interactions between the gut-brain axis in ASD severity. However, the improvements seen in the behavior and communication of ASD patients showed regression at a later follow-up, suggesting only a temporary benefit to antibiotic treatments.<ref name=Sandler/> | |||
<br><br> | |||
A later study also supports beneficial outcomes of antibiotic treatments for ASD. Geier et al.,<ref name=Geier/> administered levocarnitine treatment over a three month period to individuals diagnosed with autism spectrum disorder in a randomized, double-blind clinical trial. This treatment showed significant improvement in ASD symptoms as well as physical improvement in the loss of fat mass and gain of muscle mass.<ref name=Geier>Geier DA, Kern JK, Davis G, King PG, Adams JB, Young JL, et al. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit. 2011 Jun 1;17(6):PI15–23.</ref> Although the results of this study are promising, later follow-up on the subjects was not conducted, so it is unclear whether these improvements are only temporary like what was found by Sandler et al. in Vancomycin treatment. Nonetheless, antibiotic treatments remain a viable option for ASD treatment but should be considered in combination with other forms of therapy. | |||
<br><br> | |||
===Microbiota Transplantation and Transfer=== | |||
Two other options for microbiota treatment in autism spectrum disorder are fecal microbiota transplantation (FMT) and microbiota transfer therapy (MTT). In FMT, filtrate stool from a healthy donor is injected into a patient to restore their own gut microbiome to a healthy state.<ref name=Mangiola>Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016 Jan 7;22(1):361–8.</ref> FMT has been used to treat various GI issues, such as food poisoning and severe diarrhea, <i>Clostridium difficile</i> infections, and irritable bowel syndrome (IBS).<ref name=Zhang>Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should We Standardize the 1,700-Year-Old Fecal Microbiota Transplantation? Official journal of the American College of Gastroenterology | ACG. 2012 Nov;107(11):1755.</ref><ref name=Vrieze>Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: A safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Practice & Research Clinical Gastroenterology. 2013 Feb 1;27(1):127–37.</ref> Given the commonality of GI issues in individuals with ASD and the correlation between GI symptoms and ASD severity, FMT has promising potential as a method of therapy in treating ASD. However, the safety of FMT and possible adverse effects, such as diarrhea, abdominal cramps, belching in the short term, mild | |||
abdominal discomfort/bloating, and transient low-grade fever, need to be considered in its application to ASD.<ref name=Li/><ref name=Kelly>Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015 Jul 1;149(1):223–37.</ref> | |||
<br><br> | |||
Due to the concerns of FMT safety in treating ASD, very few studies have been conducted on it. One researcher, T. Borody, reports improvement in ASD symptoms for two children as a result of FMT treatment but there is no published study on these results.<ref name=Aroniadis>Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Current Opinion in Gastroenterology. 2013 Jan;29(1):79–84.</ref> One small clinical trial of FMT treatment for ASD was conducted in Beijing, China through 2016 and 2017. This study found that those given the FMT showed 10.8% improvement in ASD symptoms after the first transplant and maintained this improvement after a second transplant. Additionally only mild adverse effects were reported with the treatment.<ref name=Zhao>Zhao H, Gao X, Xi L, Shi Y, Peng L, Wang C, et al. Mo1667 FECAL MICROBIOTA TRANSPLANTATION FOR CHILDREN WITH AUTISM SPECTRUM DISORDER. Gastrointestinal Endoscopy. 2019 Jun 1;89(6):AB512–3.</ref> These reports suggest FMT does have high potential for ASD treatment, but more research is required before it can be a widely accepted form of therapy. | |||
<br><br> | |||
MTT is another method of FMT. In MTT, a 2 week course of antibiotics is followed by a bowel cleanse and decreasing daily doses of FMT over 7 to 8 weeks.<ref name=Kang17>Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017 Jan 23;5(1):10.</ref> Kang et al. administered this treatment to children with ASD for very positive outcomes. Patients showed an approximately 80% decrease in GI symptoms and significant improvement in ASD symptoms that persisted for the 8 weeks after treatment in which the patients were followed, suggesting a long-term impact.<ref name=Kang17/> MTT proves to be the most promising microbiota treatment for ASD as of yet. | |||
<br><br> | |||
===Probiotics=== | |||
[[Image:ASDprobiotics.png|thumb|300px|right|<b>Figure 5:</b> Diagram of the influence of probiotics on ASD behavior and symptoms. Photo credit: Chidambaram et al., https://link.springer.com/chapter/10.1007/978-3-030-30402-7_21.]] | |||
Probiotics are live microbes that provide health benefits to their host when ingested.<ref name=Caselli>Caselli M, Cassol F, Calò G, Holton J, Zuliani G, Gasbarrini A. Actual concept of “probiotics”: Is it more functional to science or business? World J Gastroenterol. 2013 Mar 14;19(10):1527–40.</ref> Probiotics have been shown to be successful in treating many different diseases including obesity, depression, colorectal cancer, and Crohn’s disease.<ref name=Verna>Verna EC, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol. 2010 Sep 1;3(5):307–19.</ref><ref name=Verma>Verma A, Shukla G. Synbiotic (Lactobacillus rhamnosus+Lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague’Dawley rats: A long-term study. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2014 Jul 14;23.</ref><ref name=Sharma>Sharma M, Shukla G. Metabiotics: One Step ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front Microbiol [Internet]. 2016 [cited 2021 Apr 7];7. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2016.01940/full</ref><ref name=Valsecchi>Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut Microbiota and Obesity. J Clin Gastroenterol. 2016 Nov 1;50 Suppl 2, Proceedings from the 8th Probiotics, Prebio:S157–8.</ref> Probiotic treatment is a very accessible treatment as it can be done by simply consuming certain foods and no medical professional is needed. For example, eating a fermented milk product, like yogurt or cheese, that contains <i>Bifidobacterium animalis</i> | |||
subsp <i>lactis, Streptococcus thermophilus, Lactobacillus bulgaricus, </i>and <i>Lactococcus lactis</i> subsp <i>lactis</i> has been shown to alter activity in the regions of the brain that control the processing of emotions and sensations.<ref name=Tillisch>Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology. 2013 Jun 1;144(7):1394-1401.e4.</ref> Given the ability of probiotics to both alter brain chemistry and GI health, it makes the treatment ideal for ASD patients with GI issues. | |||
<br><br> | |||
Probiotics have also been shown to improve intestinal barrier function, a common GI issue in individuals with ASD. <i>Lactobacillus reuteri</i> and <i>Lactobacillus rhamnosus</i>, have been shown to decrease barrier permeability through increased expression of tight junction proteins and improved mucus layer integrity.<ref name=Ulluwishewa>Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. The Journal of Nutrition. 2011 May 1;141(5):769–76.</ref><ref name=Dicksved>Dicksved J, Schreiber O, Willing B, Petersson J, Rang S, Phillipson M, et al. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLOS ONE. 2012 Sep 27;7(9):e46399.</ref> <i>Bacteroides fragilis</i> was shown to both reduce intestinal barrier permeability and improve communicative, sensorimotor, and anxiety-like behaviors in a model mouse system for studying ASD and other neurodevelopmental conditions.<ref name=Hsiao/> | |||
<br><br> | |||
Several other studies have also found probiotics that improve ASD symptoms. Parracho et al.<ref name=Parracho10>Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics & Prebiotics. 2010 May 1;5(2):69.</ref> delivered a three week course of probiotic <i>Lactobacillus plantarum</i> to patients with ASD. After treatment, the patients displayed improved ASD symptoms in destructive and antisocial behaviour, anxiety, and communication problems.<ref name=Parracho10/> Another study delivered a 2 month course of probiotic <i>L. acidophilus</i> to ASD patients and found improved ASD symptoms in concentration and abilty to follow directions.<ref name=Kaluzna>Kałużna-Czaplińska J, Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012 Feb 1;28(2):124–6.</ref> In a study examining the effectiveness of combining both antibiotic and probiotic treatment, an 8 week course of vancomycin was followed by a 4 week course of probiotic <i>L. acidophilus, L. bulgaricus</i> and <i>B. bifidum</i>, and individuals exhibited improved ASD symptoms in communication and behavioral patterns after treatment.<ref name=Sandler/> With such positive outcomes and no apparent adverse effects, probiotic treatments stand as a strong option for ASD treatment. | |||
<br><br> | |||
== | ===Diet=== | ||
ASD-related feeding concerns and selective eating habits are one of the contributors to the abnormal gut microbiome in individuals with autism spectrum disorder. Adjusting diet is a way that the gut microbiota could be adjusted. The introduction of more omega-3 fatty acids to the diet of children with ASD was shown to be one beneficial dietary change. In a 12 week trial, omega-3 fatty acid supplements were associated with significant improvement in social and behavioral ASD symptoms.<ref name=Ooi>Ooi YP, Weng S-J, Jang LY, Low L, Seah J, Teo S, et al. Omega-3 fatty acids in the management of autism spectrum disorders: findings from an open-label pilot study in Singapore. European Journal of Clinical Nutrition. 2015 Aug;69(8):969–71.</ref> Several studies have shown that gluten-free and casein-free diets are associated with improved behavior, social, and physiological related ASD symptoms.<ref name=Knivsberg>Knivsberg AM, Reichelt KL, HØien T, NØdland M. A Randomised, Controlled Study of Dietary Intervention in Autistic Syndromes. Nutritional Neuroscience. 2002 Jan 1;5(4):251–61.</ref><ref name=Millward>Millward C, Ferriter M, Calver SJ, Connell‐Jones GG. Gluten‐ and casein‐free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews [Internet]. 2008 [cited 2021 Apr 7];(2). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003498.pub3/abstract</ref><ref name=Pennesi>Pennesi CM, Klein LC. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutritional Neuroscience. 2012 Mar 1;15(2):85–91.</ref> A high-fat and low-carbohydrate keto diet has also been shown to increase sociability and communication as well as reduce repetitive behaviors and lower gut microbial abundance.<ref name=Ruskin>Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Jr MK, et al. Ketogenic Diet Improves Core Symptoms of Autism in BTBR Mice. PLOS ONE. 2013 Jun 5;8(6):e65021.</ref><ref name=Castro>Castro K, Baronio D, Perry IS, Riesgo R dos S, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutritional Neuroscience. 2017 Jul 3;20(6):343–50.</ref> | |||
==Conclusion== | ==Conclusion== | ||
As awareness and understanding of autism spectrum disorder increases, the number of individuals diagnosed with the condition has risen. This has made research and treatments for ASD more relevant and important. GI issues are a common comorbidity in individuals with ASD. With interactions between the central nervous system and gut microbiome in the gut-brain axis, addressing these intestinal issues can help improve the neurological symptoms related to ASD. The composition of the gut microbiome in ASD patients differs from that of the average person. Some of these bacteria that are more abundant within the ASD microbiome are known to release neurotoxins that likely contribute to worsened ASD symptom severity. There are many possible factors that may contribute to the development of ASD and the abnormal gut microbiome associated with the condition, and much more research is needed to pinpoint the exact causes. Nonetheless, treatments and therapies to address the ASD microbiome and GI issues are available. Research has shown successful outcomes in a variety treatments including antibiotics, probiotics, fecal microbiota transplants, and dietary changes. Although these treatments are certainly no cure to the disorder, they can significantly improve the severity of ASD and gastrointestinal problems. | |||
==References== | ==References== | ||
<references /> | <references /> | ||
<br><br>Authored for BIOL 238 Microbiology, taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2021, [http://www.kenyon.edu/index.xml Kenyon College]. | <br><br>Authored for BIOL 238 Microbiology, taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2021, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
Latest revision as of 01:43, 8 April 2021
Introduction
By Bailey Fitzgerald
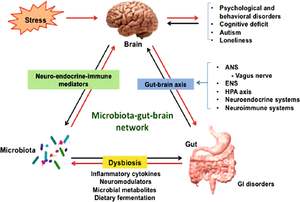
A healthy adult human intestine harbors billions of bacteria of over 1000 different species.[1][2] These bacteria play a large role in human health through digestion and aiding in immune system development.[2] However, these bacteria can also have a direct influence on their human host’s behavior. Gut microbes have the ability to bidirectionally communicate with the central nervous system and influence both emotional and cognitive centers of the brain.[3] This makes maintenance of the gut microbiome especially important in people with a variety of neurological conditions, including autism spectrum disorder (ASD).
Autism spectrum disorder is a neurological and developmental disorder characterized by deficits in social communication and social interaction, repetitive patterns of behavior or activities, and restricted interests.[4] The CDC estimates that approximately 1 in every 54 children in the United States is affected by autism spectrum disorder.[5] ASD can range in severity from very high-functioning (formerly known as Asperger’s syndrome) in which the individual is able to go about daily life with little to no assistance to low-functioning individuals that need assistance with basic care and needs, or anywhere in between. It is also common to have other diseases and conditions along with autism spectrum disorder. These comorbidities can include sleep disorders, seizure disorders, gastrointestinal disorders, metabolic disorders, and hormonal dysfunction.[6]
Gastrointestinal (GI) issues, such as frequent abdominal pain, gaseousness, diarrhea, constipation or pain on stooling, are a very common comorbidity in ASD patients.[7][8] Studies have found that 23% to 70% of people with ASD suffer from gastrointestinal dysfunction, with the wide range attributed to variances in survey methods.[8] Additionally, the prevalence of more gastrointestinal symptoms was strongly correlated with the severity of autism in patients.[9] These gastrointestinal issues can be attributed to a disturbance in normal gut flora composition.[9] Although the cause of the abnormal gut microbiome in patients with ASD is not certain, researchers hypothesize that it could be due to atypical eating habits.[10] Thus, therapies targeting the gut microbiome could have potential in treating and managing autism spectrum disorders. Because the exact causes of ASD and the mechanisms behind the disorder are difficult to identify, there are very few effective therapies available.[11] This makes the potential of microbiome treatments for ASD even more promising and hopeful for managing the disorder.
Composition of the ASD Microbiome

The microbiome of individuals with autism spectrum disorder differs from that of the average person. Firstly, the intestinal environment itself is abnormal in patients with ASD compared to the typical person. Approximately one third of patients with ASD displayed a higher percentage of abnormal permeability in their intestines compared to the approximately 5% of abnormal permeability in the control population.[12] This increased intestinal permeability causes an increased antigenic load from the GI tract. This allows more chemical signals originating from the GI tract to enter the circulation and then cross over the blood-brain barrier to deliver these chemical signals to the brain where they induce immune responses.[7] Therefore, the microbiota of ASD patients may have even more influence through the gut-brain axis than in the average person.
Patients with ASD have different species of bacteria prevalent in their gut microbiome. The maternal immune activation (MIA) mouse model is an ideal model for studying ASD as it displays many features of the disorder. One study found that offspring of mothers with MIA had more Porphyromonadaceae, Prevotellaceae, unclassified Bacteroidales, and Lachnospiraceae bacterias, whereas control offspring had more Ruminococcaceae, Erysipelotrichaceae, and Alcaligenaceae bacterias.[13] Another model system for studying ASD can be created by inducing autistic-like behaviors in offspring of mice through the administration of valproic acid in pregnant mice. In all offspring of the VPA treated mice, Bacteroidetes and Desulfovibrionales increased in prevalence while Firmicutes decreased. Additionally, male VPA offspring possessed more Alistipes, Enterorhabdus, Mollicutes and Erysipelotrichalis bacteria compared to their female siblings.[14]
Analyses of the microbiota within humans with ASD have been mostly consistent with the findings of these animal models. The microbiome of children with autism spectrum disorder has been shown to be less diverse overall and contains lower levels of Bifidobacterium and Firmicutes while the prevalence of Lactobacillus, Clostridium, Bacteroidetes, Desulfovibrio, Caloramator, and Sarcina bacterias are higher.[15][16][9][17][18][7] Autistic children specifically with GI symptoms had lower prevalence of Prevotella, Coprococcus, and unclassified Veillonellaceae bacteria. Given that these bacteria are versatile carbohydrate-degrading and/or fermenting bacteria, it would suggest that these differences could be attributed to atypical diet and feeding behaviors associated with ASD; however, the study showed no correlation between diet patterns and microbial diversity or abundance.[19]
The increased abundance of Clostridium bacteria in the gut microbiome of individuals with ASD is cause for concern. Clostridium bacteria are known to be toxin-producers, their products of which include neurotoxins that could be harmful to individuals with ASD through the gut-brain axis.[20] Additionally, this bacteria may contribute to gut dysfunction and GI symptoms.[21] Given that increased GI symptoms and ASD severity show positive correlation, Clostridium bacteria may be associated with the development of certain autistic characteristics.[9][21]
Development of the Gut Microbiota and ASD
Autism spectrum disorder has been observed to have both genetic and environmental factors to its development.[22] Environmental factors specifically affecting the composition and development of the gut microbiome may have an effect on the development of autism spectrum disorder. The most crucial environment in ASD development is the prenatal conditions within their mother’s womb. This is largely determined by maternal health.
Several studies have found a significant connection between maternal obesity, gestational diabetes (GDM), and pregestational diabetes (PGDM) in increased risk of ASD.[23][24][25] Having just one of these conditions during pregnancy is associated with a 1.5 to 2 times increased risk factor for ASD. Whereas when obesity and GDM or obesity and PGDM were both present in mothers, ASD risk showed approximately 2 to 4 times increase.[23][25]
The maternal immune system and responses can also play a factor in ASD development. Mothers of children with ASD have been shown to possess different antibodies than mothers of neurotypical children.[26] When reacted with rat prenatal (PN18) brain proteins, mothers of children with ASD and children with ASD showed one of two patterns while control mothers and their neurotypical children typically showed no reactivity (Figure 3). This suggests these antibodies are pathogenic to fetal brains in autism.[26] Maternal antibodies have also been shown to react with lymphocytes in children with ASD which further suggests reactivity with other tissues in the developing embryo.[27]
A mother’s own microbiota can play a crucial role in fetal development. If the microbiome becomes imbalanced and pathogenic microbes are able to dominate, it can mean more than just illness for an expecting mother. Viral infections during the first trimester and bacterial infections during the second trimester have been associated with greater risk of ASD.[28] Additionally, delivery method can shape the microbiome of newborn babies. Infants delivered vaginally possess gut bacteria very similar to their mother’s vaginal microbiota, mostly composed of Lactobacillus, Prevotella, and Sneathis spp.; infants delivered via Cesarean section have a gut microbiome similar to the composition of their mother’s skin microbiota, mainly consisting of Staphylococcus, Corynebacterium, and Propionibacterium spp.[29] Autism spectrum disorder risk for infants delivered through Cesarean section has been found to be higher than in infants delivered vaginally.[30] Additionally, antibiotics can restructure the composition of the gut microbiome. In children with ASD, the use of antibiotics is much more common than in their neurotypical counterparts.[7] This leads to further imbalance to their already abnormal microbiome composition.
After birth, diet plays a critical role in shaping and maintaining the human gut microbiome. Risk of ASD has been observed to increase for infants that are not breastfed and decreases with longer periods of breastfeeding.[31] However, complications in breastfeeding infants with ASD, such as difficulty with the child’s engagement in the feeding process and temperamental or behavioral issues associated with ASD, may also be to blame for this correlation.[10] Later in life, feeding issues associated with ASD may contribute to an imbalanced gut microbiome. 90% of children with ASD display feeding related concerns, the most common of which is food selectivity.[32][10] This selectivity and imbalanced diet leads to many health concerns such as nutritional deficiencies and GI conditions.[10]
Microbiome Treatments for ASD
Antibiotics
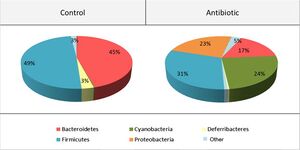
Antibiotics may be a viable option for treating neurological conditions, like autism spectrum disorder. The use of antibiotic treatment in mice was shown to have impacts on anxiety and cognitive behaviors through the alteration of gut-brain communication neuromodulators.[33] Desbonnet et al. administered treatment that restructured the gut microbiome and reduced the richness of bacterial species (Figure 4). This restructured microbiome resulted in altered dynamics of the tryptophan metabolic pathway, and significantly reduced BDNF, oxytocin and vasopressin expression in the brain; changes in these neurochemical levels showed association with reduction of anxiety, non-spatial memory, and performance in the social transmission of food preference.[33] Overall, this study shows evidence of interactions between antibiotic treatments and neurological processes, solidifying antibiotics as a possible therapy for neurological disorders.
Antibiotic treatment has been shown to be beneficial in treating ASD patients that also suffer from GI symptoms. After a short course of oral vancomycin treatment, children with ASD exhibited both reduced diarrhea and improved behavior and communication.[34] These results suggest the treatment caused a reduction of pathogenic bacteria in the gut microbiome and provides further evidence of interactions between the gut-brain axis in ASD severity. However, the improvements seen in the behavior and communication of ASD patients showed regression at a later follow-up, suggesting only a temporary benefit to antibiotic treatments.[34]
A later study also supports beneficial outcomes of antibiotic treatments for ASD. Geier et al.,[35] administered levocarnitine treatment over a three month period to individuals diagnosed with autism spectrum disorder in a randomized, double-blind clinical trial. This treatment showed significant improvement in ASD symptoms as well as physical improvement in the loss of fat mass and gain of muscle mass.[35] Although the results of this study are promising, later follow-up on the subjects was not conducted, so it is unclear whether these improvements are only temporary like what was found by Sandler et al. in Vancomycin treatment. Nonetheless, antibiotic treatments remain a viable option for ASD treatment but should be considered in combination with other forms of therapy.
Microbiota Transplantation and Transfer
Two other options for microbiota treatment in autism spectrum disorder are fecal microbiota transplantation (FMT) and microbiota transfer therapy (MTT). In FMT, filtrate stool from a healthy donor is injected into a patient to restore their own gut microbiome to a healthy state.[36] FMT has been used to treat various GI issues, such as food poisoning and severe diarrhea, Clostridium difficile infections, and irritable bowel syndrome (IBS).[37][38] Given the commonality of GI issues in individuals with ASD and the correlation between GI symptoms and ASD severity, FMT has promising potential as a method of therapy in treating ASD. However, the safety of FMT and possible adverse effects, such as diarrhea, abdominal cramps, belching in the short term, mild
abdominal discomfort/bloating, and transient low-grade fever, need to be considered in its application to ASD.[7][39]
Due to the concerns of FMT safety in treating ASD, very few studies have been conducted on it. One researcher, T. Borody, reports improvement in ASD symptoms for two children as a result of FMT treatment but there is no published study on these results.[40] One small clinical trial of FMT treatment for ASD was conducted in Beijing, China through 2016 and 2017. This study found that those given the FMT showed 10.8% improvement in ASD symptoms after the first transplant and maintained this improvement after a second transplant. Additionally only mild adverse effects were reported with the treatment.[41] These reports suggest FMT does have high potential for ASD treatment, but more research is required before it can be a widely accepted form of therapy.
MTT is another method of FMT. In MTT, a 2 week course of antibiotics is followed by a bowel cleanse and decreasing daily doses of FMT over 7 to 8 weeks.[42] Kang et al. administered this treatment to children with ASD for very positive outcomes. Patients showed an approximately 80% decrease in GI symptoms and significant improvement in ASD symptoms that persisted for the 8 weeks after treatment in which the patients were followed, suggesting a long-term impact.[42] MTT proves to be the most promising microbiota treatment for ASD as of yet.
Probiotics
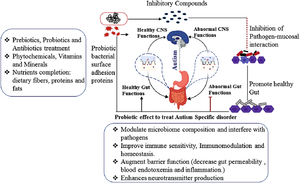
Probiotics are live microbes that provide health benefits to their host when ingested.[43] Probiotics have been shown to be successful in treating many different diseases including obesity, depression, colorectal cancer, and Crohn’s disease.[44][45][46][47] Probiotic treatment is a very accessible treatment as it can be done by simply consuming certain foods and no medical professional is needed. For example, eating a fermented milk product, like yogurt or cheese, that contains Bifidobacterium animalis
subsp lactis, Streptococcus thermophilus, Lactobacillus bulgaricus, and Lactococcus lactis subsp lactis has been shown to alter activity in the regions of the brain that control the processing of emotions and sensations.[48] Given the ability of probiotics to both alter brain chemistry and GI health, it makes the treatment ideal for ASD patients with GI issues.
Probiotics have also been shown to improve intestinal barrier function, a common GI issue in individuals with ASD. Lactobacillus reuteri and Lactobacillus rhamnosus, have been shown to decrease barrier permeability through increased expression of tight junction proteins and improved mucus layer integrity.[49][50] Bacteroides fragilis was shown to both reduce intestinal barrier permeability and improve communicative, sensorimotor, and anxiety-like behaviors in a model mouse system for studying ASD and other neurodevelopmental conditions.[13]
Several other studies have also found probiotics that improve ASD symptoms. Parracho et al.[51] delivered a three week course of probiotic Lactobacillus plantarum to patients with ASD. After treatment, the patients displayed improved ASD symptoms in destructive and antisocial behaviour, anxiety, and communication problems.[51] Another study delivered a 2 month course of probiotic L. acidophilus to ASD patients and found improved ASD symptoms in concentration and abilty to follow directions.[52] In a study examining the effectiveness of combining both antibiotic and probiotic treatment, an 8 week course of vancomycin was followed by a 4 week course of probiotic L. acidophilus, L. bulgaricus and B. bifidum, and individuals exhibited improved ASD symptoms in communication and behavioral patterns after treatment.[34] With such positive outcomes and no apparent adverse effects, probiotic treatments stand as a strong option for ASD treatment.
Diet
ASD-related feeding concerns and selective eating habits are one of the contributors to the abnormal gut microbiome in individuals with autism spectrum disorder. Adjusting diet is a way that the gut microbiota could be adjusted. The introduction of more omega-3 fatty acids to the diet of children with ASD was shown to be one beneficial dietary change. In a 12 week trial, omega-3 fatty acid supplements were associated with significant improvement in social and behavioral ASD symptoms.[53] Several studies have shown that gluten-free and casein-free diets are associated with improved behavior, social, and physiological related ASD symptoms.[54][55][56] A high-fat and low-carbohydrate keto diet has also been shown to increase sociability and communication as well as reduce repetitive behaviors and lower gut microbial abundance.[57][58]
Conclusion
As awareness and understanding of autism spectrum disorder increases, the number of individuals diagnosed with the condition has risen. This has made research and treatments for ASD more relevant and important. GI issues are a common comorbidity in individuals with ASD. With interactions between the central nervous system and gut microbiome in the gut-brain axis, addressing these intestinal issues can help improve the neurological symptoms related to ASD. The composition of the gut microbiome in ASD patients differs from that of the average person. Some of these bacteria that are more abundant within the ASD microbiome are known to release neurotoxins that likely contribute to worsened ASD symptom severity. There are many possible factors that may contribute to the development of ASD and the abnormal gut microbiome associated with the condition, and much more research is needed to pinpoint the exact causes. Nonetheless, treatments and therapies to address the ASD microbiome and GI issues are available. Research has shown successful outcomes in a variety treatments including antibiotics, probiotics, fecal microbiota transplants, and dietary changes. Although these treatments are certainly no cure to the disorder, they can significantly improve the severity of ASD and gastrointestinal problems.
References
- ↑ Maukonen J, Saarela M. Human gut microbiota: does diet matter? Proceedings of the Nutrition Society. 2015 Feb;74(1):23–36.
- ↑ 2.0 2.1 Bull MJ, Plummer NT. Part 1: The Human Gut Microbiome in Health and Disease. Integr Med (Encinitas). 2014 Dec;13(6):17–22.
- ↑ Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
- ↑ American Psychiatric Association, American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013.
- ↑ CDC. Autism and Developmental Disabilities Monitoring (ADDM) Network | CDC [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2021 Apr 7]. Available from: https://www.cdc.gov/ncbddd/autism/addm.html
- ↑ Bauman ML. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics. 2010 Jul;7(3):320–7.
- ↑ 7.0 7.1 7.2 7.3 7.4 Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Front Cell Neurosci [Internet]. 2017 [cited 2021 Mar 17];11. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2017.00120/full?fbclid=IwAR04gQH8jD4LVLXEJnl7MfXsCyDR7USEauiGGIc1BXJY48GTn2L5GQg-Hk0
- ↑ 8.0 8.1 Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J Autism Dev Disord. 2014 May 1;44(5):1117–27.
- ↑ 9.0 9.1 9.2 9.3 Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterology. 2011 Mar 16;11(1):22.
- ↑ 10.0 10.1 10.2 10.3 Mulle JG, Sharp WG, Cubells JF. The Gut Microbiome: A New Frontier in Autism Research. Curr Psychiatry Rep. 2013 Feb 1;15(2):1–9.
- ↑ Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Molecular Psychiatry. 2012 Apr;17(4):389–401.
- ↑ de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the Intestinal Barrier in Patients With Autism Spectrum Disorders and in Their First-degree Relatives. Journal of Pediatric Gastroenterology and Nutrition. 2010 Oct;51(4):418–24.
- ↑ 13.0 13.1 Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013 Dec 19;155(7):1451–63.
- ↑ de Theije CGM, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain, Behavior, and Immunity. 2014 Mar 1;37:197–206.
- ↑ Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E, et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clinical Infectious Diseases. 2002 Sep 1;35(Supplement_1):S6–16.
- ↑ Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010 Aug 1;16(4):444–53.
- ↑ 1. Finegold SM. Desulfovibrio species are potentially important in regressive autism. Medical Hypotheses. 2011 Aug 1;77(2):270–4.
- ↑ De Angelis M, Piccolo M, Vannini L, Siragusa S, Giacomo AD, Serrazzanetti DI, et al. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLOS ONE. 2013 Oct 9;8(10):e76993.
- ↑ Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLOS ONE. 2013 Jul 3;8(7):e68322.
- ↑ Hatheway CL. Toxigenic clostridia. Clinical Microbiology Reviews. 1990 Jan 1;3(1):66–98.
- ↑ 21.0 21.1 Parracho HM, Bingham MO, Gibson GR, McCartney A. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of Medical Microbiology. 2005;54(10):987–91.
- ↑ Hallmayer J. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011 Nov 1;68(11):1095.
- ↑ 23.0 23.1 Connolly N, Anixt J, Manning P, Lin DP-I, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder—An analysis of electronic medical records and linked birth data. Autism Research. 2016;9(8):829–37.
- ↑ Jo H, Eckel SP, Chen J-C, Cockburn M, Martinez MP, Chow T, et al. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environment International. 2019 Dec 1;133:105110.
- ↑ 25.0 25.1 Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics [Internet]. 2016 Feb 1 [cited 2021 Apr 6];137(2). Available from: https://pediatrics.aappublications.org/content/137/2/e20152206
- ↑ 26.0 26.1 Zimmerman AW, Connors SL, Matteson KJ, Lee L-C, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity. 2007 Mar 1;21(3):351–7.
- ↑ Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, et al. Detection of Maternal Antibodies in Infantile Autism. Journal of the American Academy of Child & Adolescent Psychiatry. 1990 Nov 1;29(6):873–7.
- ↑ Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2010 Dec;40(12):1423–30.
- ↑ Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences. 2010 Jun 29;107(26):11971–5.
- ↑ Curran EA, O’Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, et al. Research Review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Journal of Child Psychology and Psychiatry. 2015;56(5):500–8.
- ↑ Al-Farsi YM, Al-Sharbati MM, Waly MI, Al-Farsi OA, Al-Shafaee MA, Al-Khaduri MM, et al. Effect of suboptimal breast-feeding on occurrence of autism: A case–control study. Nutrition. 2012 Jul 1;28(7):e27–32.
- ↑ Ledford JR, Gast DL. Feeding Problems in Children With Autism Spectrum Disorders: A Review. Focus Autism Other Dev Disabl. 2006 Aug 1;21(3):153–66.
- ↑ 33.0 33.1 Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015 Aug;48:165–73.
- ↑ 34.0 34.1 34.2 Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen M-L, et al. Short-Term Benefit From Oral Vancomycin Treatment of Regressive-Onset Autism. J Child Neurol. 2000 Jul 1;15(7):429–35.
- ↑ 35.0 35.1 Geier DA, Kern JK, Davis G, King PG, Adams JB, Young JL, et al. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit. 2011 Jun 1;17(6):PI15–23.
- ↑ Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016 Jan 7;22(1):361–8.
- ↑ Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should We Standardize the 1,700-Year-Old Fecal Microbiota Transplantation? Official journal of the American College of Gastroenterology | ACG. 2012 Nov;107(11):1755.
- ↑ Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: A safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Practice & Research Clinical Gastroenterology. 2013 Feb 1;27(1):127–37.
- ↑ Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015 Jul 1;149(1):223–37.
- ↑ Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Current Opinion in Gastroenterology. 2013 Jan;29(1):79–84.
- ↑ Zhao H, Gao X, Xi L, Shi Y, Peng L, Wang C, et al. Mo1667 FECAL MICROBIOTA TRANSPLANTATION FOR CHILDREN WITH AUTISM SPECTRUM DISORDER. Gastrointestinal Endoscopy. 2019 Jun 1;89(6):AB512–3.
- ↑ 42.0 42.1 Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017 Jan 23;5(1):10.
- ↑ Caselli M, Cassol F, Calò G, Holton J, Zuliani G, Gasbarrini A. Actual concept of “probiotics”: Is it more functional to science or business? World J Gastroenterol. 2013 Mar 14;19(10):1527–40.
- ↑ Verna EC, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol. 2010 Sep 1;3(5):307–19.
- ↑ Verma A, Shukla G. Synbiotic (Lactobacillus rhamnosus+Lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1,2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague’Dawley rats: A long-term study. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2014 Jul 14;23.
- ↑ Sharma M, Shukla G. Metabiotics: One Step ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front Microbiol [Internet]. 2016 [cited 2021 Apr 7];7. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2016.01940/full
- ↑ Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut Microbiota and Obesity. J Clin Gastroenterol. 2016 Nov 1;50 Suppl 2, Proceedings from the 8th Probiotics, Prebio:S157–8.
- ↑ Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology. 2013 Jun 1;144(7):1394-1401.e4.
- ↑ Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. The Journal of Nutrition. 2011 May 1;141(5):769–76.
- ↑ Dicksved J, Schreiber O, Willing B, Petersson J, Rang S, Phillipson M, et al. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLOS ONE. 2012 Sep 27;7(9):e46399.
- ↑ 51.0 51.1 Parracho HM, Gibson GR, Knott F, Bosscher D, Kleerebezem M, McCartney AL. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. International Journal of Probiotics & Prebiotics. 2010 May 1;5(2):69.
- ↑ Kałużna-Czaplińska J, Błaszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition. 2012 Feb 1;28(2):124–6.
- ↑ Ooi YP, Weng S-J, Jang LY, Low L, Seah J, Teo S, et al. Omega-3 fatty acids in the management of autism spectrum disorders: findings from an open-label pilot study in Singapore. European Journal of Clinical Nutrition. 2015 Aug;69(8):969–71.
- ↑ Knivsberg AM, Reichelt KL, HØien T, NØdland M. A Randomised, Controlled Study of Dietary Intervention in Autistic Syndromes. Nutritional Neuroscience. 2002 Jan 1;5(4):251–61.
- ↑ Millward C, Ferriter M, Calver SJ, Connell‐Jones GG. Gluten‐ and casein‐free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews [Internet]. 2008 [cited 2021 Apr 7];(2). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003498.pub3/abstract
- ↑ Pennesi CM, Klein LC. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutritional Neuroscience. 2012 Mar 1;15(2):85–91.
- ↑ Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Jr MK, et al. Ketogenic Diet Improves Core Symptoms of Autism in BTBR Mice. PLOS ONE. 2013 Jun 5;8(6):e65021.
- ↑ Castro K, Baronio D, Perry IS, Riesgo R dos S, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutritional Neuroscience. 2017 Jul 3;20(6):343–50.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2021, Kenyon College.
