HIV-Derived Lentiviral Vectors and Their Use as Gene Therapy Agents Against Human Immunodeficiency Virus: Difference between revisions
No edit summary |
|||
| Line 81: | Line 81: | ||
Anderson ''et al'' (2009) have developed a vector system that targets both host and viral genes to successfully confer HIV resistance. The group transduced hematopoietic progenitor/stem cells (HPSC) ''ex vivo'', which differentiate into all cell types that HIV infects (including CD4+ T lymphocytes), with three different therapeutic genes, thereby producing a whole line of HIV-immune cells [1]. Once transduced, these cells can then be re-introduced into the HIV positive individual, where HIV naturally selects for the survival of these HIV-immune transgenic cells [1]. <br><br> | Anderson ''et al'' (2009) have developed a vector system that targets both host and viral genes to successfully confer HIV resistance. The group transduced hematopoietic progenitor/stem cells (HPSC) ''ex vivo'', which differentiate into all cell types that HIV infects (including CD4+ T lymphocytes), with three different therapeutic genes, thereby producing a whole line of HIV-immune cells [1]. Once transduced, these cells can then be re-introduced into the HIV positive individual, where HIV naturally selects for the survival of these HIV-immune transgenic cells [1]. <br><br> | ||
The lentiviral vector created contained three anti-HIV therapeutic agents that function to interfere with infection at different stages of the replication cycle. The transgene that acts first in the replication cycle is the CCR5 short hairpin RNA (shRNA) that acts to prevent entry of the virus into the host cell [1]. CCR5 is a white blood cell surface protein present on host cells that interact with viral glycoproteins to initiate transduction [1]. The ''CCR5'' gene was proposed as a target for HIV gene therapy after the discovery that people homozygous and heterozygous for a ''CCR5 Δ32'' mutation are naturally immune to HIV, and individuals lacking any ''CCR5'' gene copies are functionally normal [4]. When an HIV patient was given transplants of T-cells homozygous for the ''Δ32'' mutation his HIV reached undetectable amounts [4, 1]. Furthermore, the ''CCR5'' gene is a good candidate for gene therapy because it is a host gene, and thus will not undergo quick mutation and resistance selection like viral genes [4]. The ''CCR5'' shRNA in the vector construct acts similarly to miRNA interference, resulting in the silencing of the ''CCR''5 gene. <br><br> | The lentiviral vector created contained three anti-HIV therapeutic agents that function to interfere with infection at different stages of the replication cycle. The transgene that acts first in the replication cycle is the CCR5 short hairpin RNA (shRNA) that acts to prevent entry of the virus into the host cell [1]. CCR5 is a white blood cell surface protein present on host cells that interact with viral glycoproteins to initiate transduction [1]. The ''CCR5'' gene was proposed as a target for HIV gene therapy after the discovery that people homozygous and heterozygous for a ''CCR5 Δ32'' mutation are naturally immune to HIV, and individuals lacking any ''CCR5'' gene copies are functionally normal [4]. When an HIV patient was given transplants of T-cells homozygous for the ''Δ32'' mutation his HIV reached undetectable amounts [4, 1]. Furthermore, the ''CCR5'' gene is a good candidate for gene therapy because it is a host gene, and thus will not undergo quick mutation and resistance selection like viral genes [4]. The ''CCR5'' shRNA in the vector construct acts similarly to miRNA interference, resulting in the silencing of the ''CCR''5 gene. <br><br> | ||
The second transgene included in the vector was a chimeric human and macaque version of TRIM5α to prevent integration of the provirus [1]. Certain monkey TRIM5α isoforms prevent HIV infection by interfering with the opening of the virus protein capsid after entry [1]. The DNA encoding the monkey amino acids responsible for conferring HIV resistance were fused to human ''TRIM5α'' gene in order to prevent the protein from being rejected by the immune system [1]. <br> | The second transgene included in the vector was a chimeric human and macaque version of TRIM5α to prevent integration of the provirus [1]. Certain monkey TRIM5α isoforms prevent HIV infection by interfering with the opening of the virus protein capsid after entry [1]. The DNA encoding the monkey amino acids responsible for conferring HIV resistance were fused to human ''TRIM5α'' gene in order to prevent the protein from being rejected by the immune system [1]. <br><br> | ||
The third transgene mimics the viral transactivation response (TAR) element | |||
[[Image:tar.png|thumb|300px|right|Figure 9. TAR element binding to elongation factors. Source: Springer Images, http://www.springerimages.com/Images/RSS/1-10.1007_s11481-011-9267-6-2]] | |||
The third transgene mimics the viral transactivation response (TAR) element is controlled by the virulence ''tat'' gene to preventing transcription of the viral genome [1]. Functional TAR elements are recruited by the ''tat'' gene to bind to elongation factors during transcription in order to catalyze the addition of nucleotides to the growing chain by polymerase enzymes [1]. The decoy TAR element used still binds to ''tat'' during transcription, but is modified to prevent transcription rather than participate in it. | |||
[[Image:tar.png|thumb|300px|center|Figure 10. Modulated construct created by Anderson ''et al'', 2009. Source: http://www.nature.com/mt/journal/v17/n12/pdf/mt2009187a.pdf]] | |||
<br><br> | <br><br> | ||
| Line 90: | Line 96: | ||
==The Future of Lentiviral Therapy== | ==The Future of Lentiviral Therapy== | ||
[[Image:HIVmemorial.png|thumb|300px|right|Figure | [[Image:HIVmemorial.png|thumb|300px|right|Figure 11. The AIDS memorial quilt displayed on the Washington D.C. Mall in 1987. Source: Encyclopedia Britannica. http://www.britannica.com/EBchecked/media/120046/The-AIDS-Memorial-Quilt-was-first-displayed-on-the-Mall]] | ||
Gene therapy first gained notoriety when Ashanti DeSilva was cured with severe combined immune deficiency (SCID), and since 2007 over 1,340 gene therapy trials have been performed in over 28 different countries [5]. While most gene therapy trials are used to target cancer, all individuals currently being treated using gene therapy are individuals with chronic conditions where gene therapy is used as a last resort [5]. While the public appears to be generally growing more accepting of the use of gene therapy, confusion and hesitation arise from the possibility of using human genetic modification not just for the treatment of genetic diseases, but for the selection of non-disease related genes as well [9]. Furthermore, many patients undergoing gene therapy are actually too young to understand how gene therapy works [9]. It has been proposed that to foster a higher public opinion of gene therapy, researchers need not educate more, but rather be more open and honest in their trials to build a public trust [9]. While the successes of gene therapy outweigh the failures, gene therapy-associated catastrophes are hard to forget, such as the activation of the ''LMO2'' oncogene in two out of eleven patients being treated for SCIDs in 2002 [2]. Fears of the consequences of gene therapy are present not only in the public, but triggered by such events in the scientific community as well. While current lentiviral vectors are significantly safer than their first-generation counterparts and advancements are being made daily, there is still much to be discovered about genetic modification. At the same time, treatments save lives and help most patients, such as those affected with hemophilia B, thalassemia, and X-linked adrenoleukodystrophy [10]. Gene therapy is most commonly used in the treatment of cancer, but offers solutions to a vast array of genetic diseases. As gene therapy trials move forward towards safer, more effective, and more reliable mechanisms, they provide more hope to the aid in chronic disorders. As gene therapy continues to report more successful results, it is highly likely that they will only become more integrated into current medicinal practices. | Gene therapy first gained notoriety when Ashanti DeSilva was cured with severe combined immune deficiency (SCID), and since 2007 over 1,340 gene therapy trials have been performed in over 28 different countries [5]. While most gene therapy trials are used to target cancer, all individuals currently being treated using gene therapy are individuals with chronic conditions where gene therapy is used as a last resort [5]. While the public appears to be generally growing more accepting of the use of gene therapy, confusion and hesitation arise from the possibility of using human genetic modification not just for the treatment of genetic diseases, but for the selection of non-disease related genes as well [9]. Furthermore, many patients undergoing gene therapy are actually too young to understand how gene therapy works [9]. It has been proposed that to foster a higher public opinion of gene therapy, researchers need not educate more, but rather be more open and honest in their trials to build a public trust [9]. While the successes of gene therapy outweigh the failures, gene therapy-associated catastrophes are hard to forget, such as the activation of the ''LMO2'' oncogene in two out of eleven patients being treated for SCIDs in 2002 [2]. Fears of the consequences of gene therapy are present not only in the public, but triggered by such events in the scientific community as well. While current lentiviral vectors are significantly safer than their first-generation counterparts and advancements are being made daily, there is still much to be discovered about genetic modification. At the same time, treatments save lives and help most patients, such as those affected with hemophilia B, thalassemia, and X-linked adrenoleukodystrophy [10]. Gene therapy is most commonly used in the treatment of cancer, but offers solutions to a vast array of genetic diseases. As gene therapy trials move forward towards safer, more effective, and more reliable mechanisms, they provide more hope to the aid in chronic disorders. As gene therapy continues to report more successful results, it is highly likely that they will only become more integrated into current medicinal practices. | ||
Revision as of 06:02, 9 May 2013
Introduction
By Audrey White
What is gene therapy?
Gene therapy is a method of treating genetic disorders in which an individual’s genes are modified to correct or prevent a disease. Depending on the genetic disease, the subject’s cells can be treated by replacement of a mutant gene with a healthy gene, deactivation of a disease gene, or introduction of a gene to fight a disease. Unlike pharmaceutical drugs, gene therapy often offers a permanent cure for individuals who have inherited diseased genes, rather than treatment to manage symptoms. One mechanism of cell transformation for gene therapy involves the use of modified viral agents to introduce or remove genes from host cells.
Why use viral agents?
Viruses are naturally occurring obligate intra-cellular infectious agents that require host cell machinery in order to replicate [12]. During replication, the virus transfects the host cell with its own DNA. Researchers have taken advantage of the efficiency of viral transformation by modifying the viral genome to carry a therapeutic gene in place of a non-essential viral gene [12]. To date, viral vectors have been used to induce pluripotent stem cells, silence genes, induce transgene expression, and confer immunization [10].
What are Lentiviruses?
For some viral genera, replication involves the insertion of the viral genome into that of the host cell. Once integrated, the viral genes are replicated and passed on to daughter cells as though part of the original host cell. One such genera Lentivirus of the Retroviridae family, who use a reverse transcriptase enzyme to produce a DNA copy of its RNA genome before inserting the DNA genome copy into the genome of the host [8]. Lentiviruses are appealing as gene therapy agents because of this permanency of transformation. Lentivuses were first proposed in 1996 as gene therapy vectors because of this ability to integrate their genome into host DNA, as well as their ability to target different cell types and infect both dividing and non-dividing cells [10]. One species of lentivirus, HIV-1, the primary cause of HIV and AIDS in humans, has been developed as a vector because of its specificity of integration at localized hotspots within the human genome, making it less likely to insert randomly and interfere with critical genes than other viral vectors, such as the murine leukemia virus [7].
Using Lentiviral Vectors to fight HIV:
One of the most encouraging advances in the use of the HIV-1 virus as a vector has actually been to fight HIV itself. Recent studies have shown that lentiviruses can be used to target key virus or host genes to confer resistance to the HIV-1 virus. This can be achieved by either targeting essential viral genes, such as replication machinery, or targeting host genes that are involved in allowing viral entry into the cell. One promising example of using HIV-1 to fight HIV involves a vector that contains three anti-HIV transgenes. Anderson et al (2009) have shown that transforming CD34+ hematopoietic progenitor cells with a CCR5 short hairpin RNA gene, a TRIM5α gene, and a transactivation response element gene, each which operate at different stages of the viral life cycle, successfully block HIV infection [1].
Overview of Lentiviral Vectors
What is the HIV-1 virus?
The HIV-1 virus is the causative agent responsible for the Acquired Immunodeficiency Syndrome (AIDS) epidemic affecting roughly 30 million people worldwide [15]. The virus is transmitted between humans most commonly through unprotected sexual intercourse [4]. Once in the body, HIV infects CD4+ lymphocytes, macrophages, and dendritic cells of the immune system [4]. Being a member of the retroviral family, HIV inserts its DNA into the host genome, thus proving to be very difficult to eradicate once the infection process has begun. This leads to the destruction of CD4+ T cells and a subsequent weakening of the immune system, causing exhaustion and allowing for opportunistic infections [4].
Brief Overview of the Life Cycle of HIV-1:
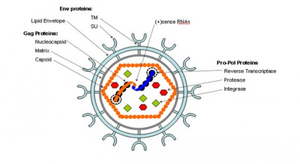
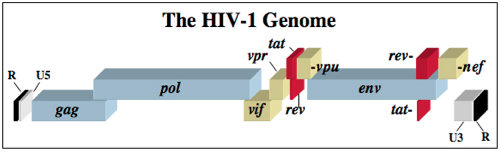
The HIV-1 virus consists of a diploid single-stranded RNA genome surrounded by a protein coat and outer phospholipid envelope (figure 1, [10]). Its genome contains three essential retroviral genes as well as well as regulatory genes that modulate host interactions (figure 2). The gag gene is responsible for glycoprotein structure, the env gene transcribes envelope proteins, and the pol gene transcribes the reverse transcriptase, responsible for producing a DNA copy of the HIV RNA genome [8]. Viral genes vif, vpr, vpu, rev, nef and tat transcribe virulence proteins [10]. All proviral genes, those that become integrated into the host (gag, pol, pro, vif, vpr, vpu, tat, and env), are bordered by two long terminal repeats (LTRs) essential for gene regulation [10]. Viral promoters and enhancers are located in the LTR U3 region [10]. The packaging signal, Ψ, is located in the 5’ end of the genome, and is responsible for packaging genes downstream of itself into the viral capsid during replication [10].
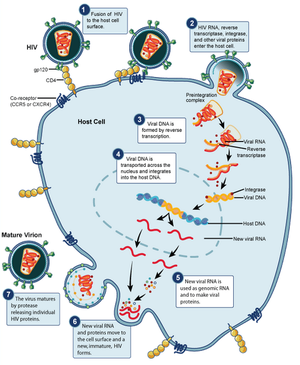
HIV begins its infection cycle when surface proteins in the phospholipid envelope interact with CD4+ T-cell CCR5 surface proteins [8, 1]. Once bound, the HIV envelope fuses with the host cell envelope, dumping the viral contents directly into the host cytoplasm [8]. Once in the cytoplasm the reverse transcriptase enzyme uses the single-stranded RNA genome as template to create a double-stranded DNA viral genome, which becomes inserted into the host genome, where it resides and safely replicates with the host cell as a provirus (figure 3) [8].
When the host cell is no longer a suitable environment, such as during times of stress, the HIV genome replicates independently, gets transcribed into RNA, packaged into a protein capsid, and acquires its phospholipid envelope by budding out of the host cell membrane [8]. HIV has evolved the ability to bud directly to adjacent cells through cell-cell junctions, thus avoiding antibodies floating in the surrounding fluids, making it particularly effective at evading the immune system [8].
History and Mutagenesis of HIV:
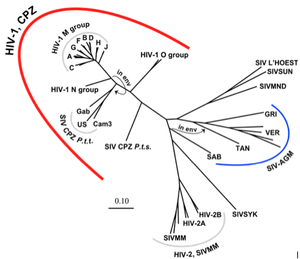
HIV is an incredibly rapidly evolving virus. It has evolved more than once, crossing the chimp-human species boundary multiple times (figure 4 [8]). While it is not usually the virus that kills the host, HIV’s invasion of T-cells weakens the immune system, allowing for opportunistic infections by other agents. The virus has the highest mutation rate known (1 in 10 base pairs) and often integrates random nucleotides when replicating, allowing the virus to continue to be unrecognizable to immune system antibodies [8]. Because the HIV genome is double stranded, the virus has two chances of acquiring a functional genome, however with such a high mutation rate most viruses are, in fact, still defective [8]. This high rate of mutation can actually be seen as a strategy; the advantage of evading the immune system is greater for HIV than the risk of producing defective offspring.
Development of HIV-1 as a Gene Therapy Agent:
HIV-1 can be used as a gene therapy vector through the replacement of virulence genes with therapeutic transgenes. These modified viruses are allowed to infect cells, where they undergo their natural infection cycle resulting in the insertion of the therapeutic genes instead of proviral genes.
Recombinant Plasmids Used for Transfection:
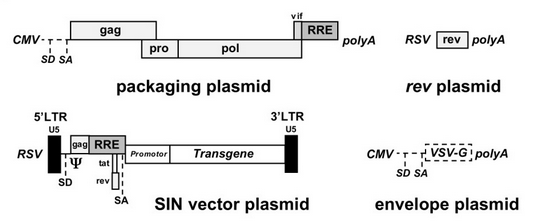
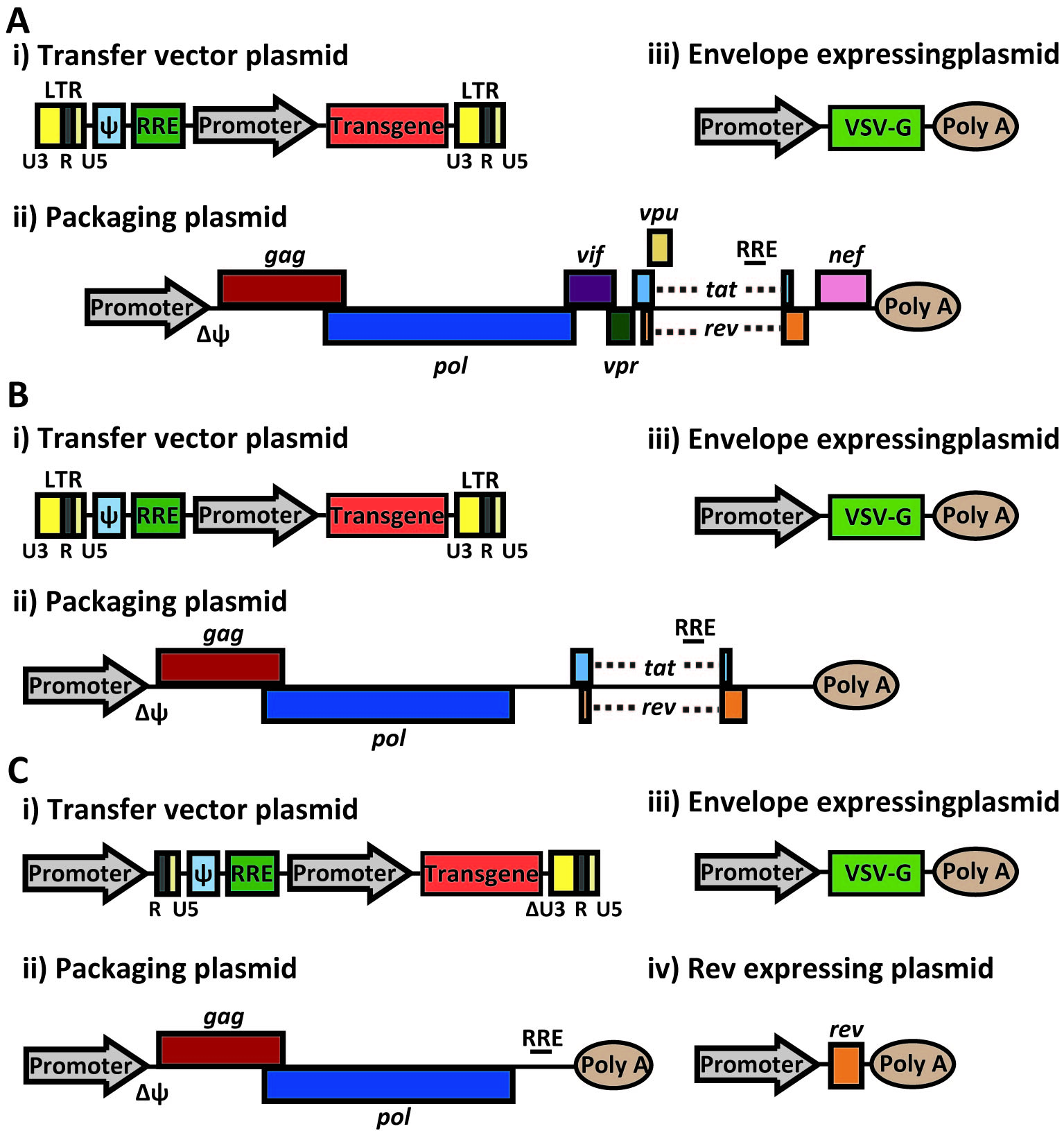
Recombinant plasmids are produced by transiently transfecting either HEK 293 or 293T cells with plasmids containing only essential viral genes and the transgene [10]. HIV particles are allowed to infect the transfected cells, where they package and thus carry only the gene of interest. This complex process is achieved through the design and organization of at least three transfected plasmids (figure 5).
The current lentiviral vectors used are third generation lentiviral vectors, which have been corrected to ensure safety and prevent the formation of replication competent lentiviuses (RCLs) through recombination between plasmids. Together, the plasmids contain only the three essential retroviral genes plus the transgene separated across the three distinct transfected plasmids. By deleting unnecessary proteins and allocating parts of the genome to different plasmids, it is extremely unlikely to acquire replication-competent lentiviruses [10].
Plasmid 1 contains a promoter and viral genes gag and pol provided in trans, both necessary to generate a functional virus [10]. Plasmid 2 contains the transgene acting in cis inserted into the original lentiviral genome after virulence genes vif, vpr, vpu, rev, tat, and nef have been removed from the original plasmid [10]. The transgene is located behind the packaging signal (Ψ) [10]. The use of Ψ is an important development in the production of lentiviral vectors, as any RNA molecule within 8-10 kb located behind Ψ will be packaged into the resulting lentiviruses providing the three essential gag, pol, and env genes are also provided in trans [12]. Because plasmid 2 is generated from the original lentiviral genome, the transgene and packaging signal are bordered by a modified version of the LTRs with the 3’ promoter deleted [10]. Plasmid 3 contains the third essential gene, env, encoding for the envelope glycoprotein, as well as a receptor-binding domain (SU) that can be altered to increase cellular tropism [10].
Regulation of Lentiviral Transduction:
The specificity of the binding of the env glycoprotein to host cell surface proteins determines which cells uptake the HIV-1 virus. Altering the glycoprotein envelope to adjust cellular tropism allows for the lentivirus to be engineered to infect cell types other than the host CD4+ T-cells. This is an incredibly useful tool as cell targeting has several benefits: there is a smaller chance of insertional mutagenesis because only essential cells are transformed, a transgene can be introduced that may be toxic to certain types of cells, and the increased specificity of the virus makes it less of a biosafety hazard [10].
Glycoproteins can be modified through many different mechanisms, such as using the glycoprotein gene of a different virus or altering the existing glycoprotein gene through chemical or genetic modification [10]. A number of different glycoproteins have been produced for lentiviral use. Transductional targeting allows for regulation before the virus integrates as a provirus or even infects host cells.
Transcriptional Targeting Using Tet-On Tet-Off System:
Often transgenes are under the control of a strong constitutive promoter and enhancer, such as that derived from the cytomegalovirus (CMV) to ensure its constant production [10]. However, the use of strong constitutive promoters increases the risks associated with insertional mutagenesis. Because the CMV promoter has the ability to activate any downstream gene, if randomly inserted in front of an oncogene the promoter may instigate devastating consequences for the patient [10]. For this reason, cell-type specific or inducible promoters are used to control gene expression within a certain time period or in response to a chemical signal [10].
One common example of both a reversible and inducible promoter that can be used in vivo is the tet-on tet-off system isolated from regulatory sequences present in tetracycline-resistant Escherichia coli [3]. The Tet operon is composed of two elements: a tetracycline repressor gene (TetR), and a tetracycline operator sequence (TetO) to which the TetR repressor protein binds [3]. The transgene is cloned into a plasmid containing the PhCMV*-1 promoter upstream of a multiple cloning site [3]. PhCMV*-1 acts as a hybrid promoter consisting of the tet-responsive elet (TRE), containing seven copies of TetO and the minimal immediate early promoter of cytomegalovirus [3]. A second plasmid is cloned containing a hybrid protein called the tet-controlled transcriptional activator (tTA), which is a fusion of the wild-type TetR to VP16 activation domain of herpes simplex virus [3]. When these two plasmids containing the modified response elements are provided in trans, the “tet-off” system transcribes the transgene in absence of tetracycline. A “tet-on” system that transcribes the transgene in the presence of tetracycline can also be produced by four amino acid changes to TetR to alter its binding characteristics, producing reverse TetR (rTetR) [3].
Lentiviral vectors that are tetracycline responsive have been developed with the “tet-off” system containing the tTA transcription factor. Any antibiotic in the tetracycline family can be used to control gene expression, with doxycycline being the most common antibiotic used [3]. The Tet system can be used in tandem with tissue specific promoters, leading to inducible expression in specific cell types.
Targeted Gene Expression through MicroRNA Silencing:
While the Tet operon allows for the control of transgene expression on a transcriptional level, transgene expression can also be controlled post-transcriptionally through microRNA silencing. MicroRNA silencing is a naturally-occurring phenomenon whereby microRNAs (miRNAs), short non-coding RNA molecules that have sequences complementary to cell mRNAs, hybridize to transcribed mRNA, causing their degradation through deadenylation or repression of translation [6].
MiRNAs hybridize to mRNA in the form of ribonucleoprotein complexes (RISC), which contain key catalytic Argonaute proteins with endonuclease properties that act as molecular scissors to break down complementary sequences [6]. Other less-understood mechanisms of RNA silencing occur when miRNAs act as physical barriers to block translation or inhibit transcription through chromatin remodeling [6]. MiRNA silencing can be used in lentiviral vectors by including an miRNA template complementary to an endogenous mutant host gene in order to silence its expression [6].
Are lentiviral vectors safe?
Lentiviral vectors currently used in gene therapy trials are the safest generation of lentiviral vectors produced, and have undergone a series of modifications to ensure their safety. Several fears in response to the use of lentiviral vectors include the fear that retroviruses will somehow undergo recombination to produce replication competent vectors that may even be capable of causing HIV. A few important measures have been taken to ensure that this does not happen, including the deletion of virulence associated genes from packaging constructs and the separation of the three essential packaging genes onto different DNA segments.
Virulence genes associated with the symptoms of AIDS, vif, vpr, vpu, rev, nef and tat, are not found in the vector constructs, leaving only the env, gag, and pol genes, without which the virus would not be able to package the transgene into the protein capsid [10]. Furthermore, the packaging sequence, Ψ, has been moved in front of the transgene and on a different construct from these essential genes, ensuring that the only segment packaged into the virus is the transgene [10]. These deletions have occurred largely on the packaging construct, who are currently considered to be third generation constructs produced from several successive deletions (figure 6). Sequence similarity between the three plasmids has been significantly reduced in order to prevent the formation of replication competent plasmids through recombination. Finally, the deletion of the 400nt region from U3 in the 3’ LTR prevents transcription of the full viral genome. Lentiviral vectors containing this deletion are therefore deemed “self-inactivating vectors” (SIN) [10].
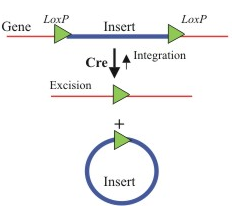
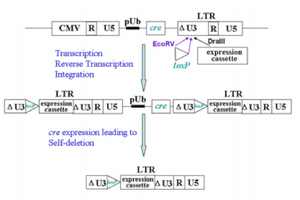
SIN vectors can be produced through genetic modification using the Cre-recombinase enzyme endogenous to bacteriophage P1. Cre-recombinase recognizes a 34 base-pair palindrome called LoxP. When two LoxP sequences exist in cis, the intermediate sequence flanked by LoxP is essentially looped out of the genome and deleted by Cre-recombinase [7]. Fang et al (2013) advanced the development of SIN vectors by using Cre-recombinase to produce vectors that do not just contain the U3 deletion, but also delete the packaging signal, primer binding site, rev response element, and cre itself gene after integration [7]. This leads to the expression of the transgene as well as removal of even more viral elements than lentiviral vectors currently being used [7]. Improvements in the safety of lentiviral vectors are constantly being made.
In addition to the formation of RCLs, there is also legitimate concern for insertional mutagenesis. Often lentiviral vectors are made with strong enhancers and promoters that have the potential to activate oncogenes, which led to the death of two out of eleven patients in 2002 being treated for X-linked SCID through retroviral gene therapy [2]. The possibility of insertional mutagenesis can be decreased by increasing the preference of integration at specific known sites. The Cre-recombinase system can also be a useful tool in specifying insertion preference sites, as the human genome contains LoxP-like sequences [13]. Cells containing LoxP-like sequences that have been transformed with a lentiviral vector containing Cre-recombinase in trans fused to the HIV accessory protein, vpr, led to the insertion of vpr at the intended LoxP site [13]. Using lentiviruses containing Cre-recombinase in trans may indeed allow for even safer gene therapy vectors.
Application of Lentiviral Vectors in HIV-Targeted Gene Therapy
Current antiretroviral medications may significantly slow the onset of AIDS, yet the viral genome is not eradicated from HIV-positive individuals, and thus current medications act only as treatments and not cures. Furthermore, the most common antiretroviral strategy, highly active antiretroviral therapy (HAART), often instigates severe side effects and readily selects for antiretroviral-resistant viruses [15]. Thus, gene therapy using HIV-1 derived lentiviral vectors are being developed to target HIV genes. The objective of HIV gene therapy is to interfere with viral replication, either by halting transduction of the virus, transcription or translation of the viral genome, or integration of the provirus [4]. This can be achieved by modifying either host or viral genes [4].
Anderson et al (2009) have developed a vector system that targets both host and viral genes to successfully confer HIV resistance. The group transduced hematopoietic progenitor/stem cells (HPSC) ex vivo, which differentiate into all cell types that HIV infects (including CD4+ T lymphocytes), with three different therapeutic genes, thereby producing a whole line of HIV-immune cells [1]. Once transduced, these cells can then be re-introduced into the HIV positive individual, where HIV naturally selects for the survival of these HIV-immune transgenic cells [1].
The lentiviral vector created contained three anti-HIV therapeutic agents that function to interfere with infection at different stages of the replication cycle. The transgene that acts first in the replication cycle is the CCR5 short hairpin RNA (shRNA) that acts to prevent entry of the virus into the host cell [1]. CCR5 is a white blood cell surface protein present on host cells that interact with viral glycoproteins to initiate transduction [1]. The CCR5 gene was proposed as a target for HIV gene therapy after the discovery that people homozygous and heterozygous for a CCR5 Δ32 mutation are naturally immune to HIV, and individuals lacking any CCR5 gene copies are functionally normal [4]. When an HIV patient was given transplants of T-cells homozygous for the Δ32 mutation his HIV reached undetectable amounts [4, 1]. Furthermore, the CCR5 gene is a good candidate for gene therapy because it is a host gene, and thus will not undergo quick mutation and resistance selection like viral genes [4]. The CCR5 shRNA in the vector construct acts similarly to miRNA interference, resulting in the silencing of the CCR5 gene.
The second transgene included in the vector was a chimeric human and macaque version of TRIM5α to prevent integration of the provirus [1]. Certain monkey TRIM5α isoforms prevent HIV infection by interfering with the opening of the virus protein capsid after entry [1]. The DNA encoding the monkey amino acids responsible for conferring HIV resistance were fused to human TRIM5α gene in order to prevent the protein from being rejected by the immune system [1].
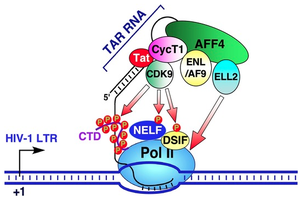
The third transgene mimics the viral transactivation response (TAR) element is controlled by the virulence tat gene to preventing transcription of the viral genome [1]. Functional TAR elements are recruited by the tat gene to bind to elongation factors during transcription in order to catalyze the addition of nucleotides to the growing chain by polymerase enzymes [1]. The decoy TAR element used still binds to tat during transcription, but is modified to prevent transcription rather than participate in it.

The lentiviral agent containing the TAR decoy, TRIM5α, and CCR5 shRNA were introduced into HPSC cells and their expression was confirmed through quantitative real-time PCR (QRT-PCR), with transgene expression being 30 to 4,000 times higher than control cells [1]. A decrease in CCR5 expression was confirmed by analyzing with FACS to ensure that there was no CCR5 on the cell surface, and was backed up by QRT-PCR [1]. Cells challenged with different strains of HIV-1 showed substantial resistance to the virus. No viral mutants resistant to the lentiviral vector were found in culture supernatants after allowing for long-term proliferation [1]. When a mix of transduced and non-transduced cells were subjected to HIV infection, there was a significant increase in the transduced cells, indicating that the HIV virus was indeed selecting for transduced cells [1].
In a later trial, when this lentiviral vector was used to introduce the three-gene construct in order to confer HIV resistance into a mouse model with a humanized immune system, the group successfully generated an immune system capable of resisting HIV infection [14]. While not yet available for human use, the construct designed by Anderson et al (2009) represents just one of the significant achievements in HIV-1 derived vector gene therapy to cure HIV positive individuals. These successes in gene therapy bring hope to the possibility that soon HIV results may not be fixed.
The Future of Lentiviral Therapy

Gene therapy first gained notoriety when Ashanti DeSilva was cured with severe combined immune deficiency (SCID), and since 2007 over 1,340 gene therapy trials have been performed in over 28 different countries [5]. While most gene therapy trials are used to target cancer, all individuals currently being treated using gene therapy are individuals with chronic conditions where gene therapy is used as a last resort [5]. While the public appears to be generally growing more accepting of the use of gene therapy, confusion and hesitation arise from the possibility of using human genetic modification not just for the treatment of genetic diseases, but for the selection of non-disease related genes as well [9]. Furthermore, many patients undergoing gene therapy are actually too young to understand how gene therapy works [9]. It has been proposed that to foster a higher public opinion of gene therapy, researchers need not educate more, but rather be more open and honest in their trials to build a public trust [9]. While the successes of gene therapy outweigh the failures, gene therapy-associated catastrophes are hard to forget, such as the activation of the LMO2 oncogene in two out of eleven patients being treated for SCIDs in 2002 [2]. Fears of the consequences of gene therapy are present not only in the public, but triggered by such events in the scientific community as well. While current lentiviral vectors are significantly safer than their first-generation counterparts and advancements are being made daily, there is still much to be discovered about genetic modification. At the same time, treatments save lives and help most patients, such as those affected with hemophilia B, thalassemia, and X-linked adrenoleukodystrophy [10]. Gene therapy is most commonly used in the treatment of cancer, but offers solutions to a vast array of genetic diseases. As gene therapy trials move forward towards safer, more effective, and more reliable mechanisms, they provide more hope to the aid in chronic disorders. As gene therapy continues to report more successful results, it is highly likely that they will only become more integrated into current medicinal practices.
References
8. Foster J W and Slonczewski J L. Microbiology: An Evolving Science. New York City: W. W. Norton Limited, 2010. Print.
9. Gottweis H. 2002. Gene therapy and the public: a matter of trust. Gene Therapy. 9 (11): 667-669.
12. Kumar P. Special focus on lentiviral vector development and applications. Gene Therapy Review.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.
