HIV Treatment: Difference between revisions
| (221 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
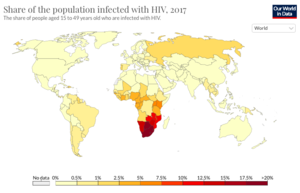
[[Image:Screen_Shot_2021-03-29_at_10.34.39_AM.png |thumb| | [[Image:Screen_Shot_2021-03-29_at_10.34.39_AM.png |thumb|300px|right|<b>Figure 1:</b> Global prevalence of HIV Infections in 2017. [https://ourworldindata.org/hiv-aids].]] | ||
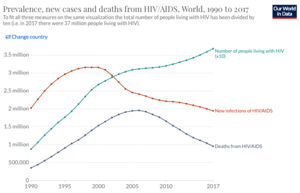
[[Image:Screen_Shot_2021-03-29_at_10.35.00_AM.png|thumb| | [[Image:Screen_Shot_2021-03-29_at_10.35.00_AM.png|thumb|300px|right|<b>Figure 2:</b> Trends in prevalence, incidence, and mortality from HIV 1990-2017. Globally, deaths and incidence are decreasing, while the total number of people with HIV continues to rise. [https://ourworldindata.org/hiv-aids].]] | ||
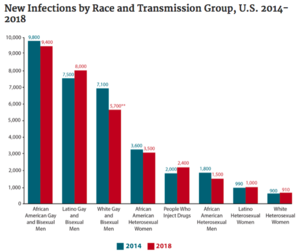
[[Image:incidence_in_US.png|thumb| | [[Image:incidence_in_US.png|thumb|300px|right|<b>Figure 3:</b> Incidence of HIV Infections in the United States from 2014-2018. [https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics].]] | ||
<br><b>By Alice Tillman</b> <br> | <br><b>By Alice Tillman</b> <br> | ||
<br><b>Human immunodeficiency virus (HIV)</b> is an RNA retrovirus which attacks the immune system of the infected individual. There are two main strains of HIV: HIV-1 and HIV-2. HIV-1 is the more prevalent and pathogenic of the two strains and therefore is the subject of most research, including this Wiki Page. Genetic sequencing techniques have revealed that HIV-1 and HIV-2 originated from separate cross-over transmission events from simian immunodeficiency virus (SIV) in chimpanzees and sooty mangabeys. HIV belongs to a type of retroviruses which are called lentiviruses. Lentiviruses infect their hosts over a very long period of time and it can take years for symptoms to manifest. Over time, HIV can progress into <b>Acquired Immunodeficiency Syndrome</b> or <b>AIDS</b>. HIV/AIDS is characterized by a decline in the number of CD4+ T-cells and an increased susceptibility to other infections. | <br><b>Human immunodeficiency virus (HIV)</b> is an RNA retrovirus which attacks the immune system of the infected individual. There are two main strains of HIV: HIV-1 and HIV-2. HIV-1 is the more prevalent and pathogenic of the two strains and therefore is the subject of most research, including this Wiki Page. Genetic sequencing techniques have revealed that HIV-1 and HIV-2 originated from separate cross-over transmission events from simian immunodeficiency virus (SIV) in chimpanzees and sooty mangabeys.<ref name=HIV_Evolution>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC164491/= Lemey, P., Pybus, O., Wang, B., Saksena, N., Salemi, M., Vandamme, A. Tracing the origin and history of the HIV-2 epidemic. <i>Proceedings of National Academy Science USA</i>. 2003, 100(11):6588-6592. doi:10.1073/pnas.0936469100]</ref> HIV belongs to a type of retroviruses which are called lentiviruses. Lentiviruses infect their hosts over a very long period of time and it can take years for symptoms to manifest. Over time, HIV can progress into <b>Acquired Immunodeficiency Syndrome</b> or <b>AIDS</b>. HIV/AIDS is characterized by a decline in the number of CD4+ T-cells and an increased susceptibility to other infections.<ref name=HIV_Infection>[https://www.nature.com/articles/nrdp201535.pdf= Deeks, S., Overbaugh, J., Phillips, A., et al. HIV infection. <i>Nature Reviews Disease Primers</I>. 2015. <b>1</b>, 15035. https://doi.org/10.1038/nrdp.2015.35]</ref> | ||
HIV is transmitted from one person to another through bodily fluids, such as blood, semen, vaginal fluid, and breast milk.<ref name=HIV_Transmission>[https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/how-is-hiv-transmitted= “How is HIV Transmitted?” HIV.gov https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/how-is-hiv-transmitted]</ref><ref name=UNAIDS>[https://www.unaids.org/en/frequently-asked-questions-about-hiv-and-aids= “HIV and AIDS – basic facts,” UNAIDS. https://www.unaids.org/en/frequently-asked-questions-about-hiv-and-aids]</ref> In order for an infection to take place, the virus must reach the bloodstream of an individual. Transmission most commonly occurs during vaginal or anal sex, through injection with needles, or from mother to child during pregnancy, labor, or breastfeeding.<ref name=HIV_Infection/><ref name=WHO_HIV>[https://www.who.int/news-room/fact-sheets/detail/hiv-aids= “HIV/AIDS,” World Health Organization. November 30, 2020. https://www.who.int/news-room/fact-sheets/detail/hiv-aids]</ref><ref name=UNAIDS/> A mother with HIV has a 15-45% chance of transmitting the virus to her child.<ref name=Mother_Child_Transmission>[https://www.who.int/hiv/topics/mtct/about/en/= “Mother-to-child transmission of HIV,” World Health Organization. https://www.who.int/hiv/topics/mtct/about/en/]</ref> HIV-positive women who take anti-viral medication during pregnancy and 4-6 weeks after childbirth have only a 5% chance of passing on the virus.<ref name=Mother_Child_Transmission/> | |||
HIV | The earliest cases of HIV were documented on June 5, 1981, when the CDC noted that five men in Los Angeles had developed pneumonia from the fungus <i>Pneumocystis jirovecii</i> in the Morbidity and Mortality Weekly Report.<ref name =HIV_Prevention>[https://www.thelancet.com/action/showPdf?pii=S0140-6736%2808%2960884-3= Merson, M., O’Malley, J., Serwadda, D., Apsuk, C. The history and challenge of HIV prevention. <i>Lancet</i>. 2008. <b>372</b>, 475-488.DOI:10.1016/S0140- 6736(08)60884-3]</ref> In the first several years of the pandemic, HIV/AIDS was mostly recorded in predominately white, gay communities in urban areas.<ref name=HIV_Infection/><ref name=HIV_Prevention/> The disease caused by HIV, AIDS, was referred to in the medical establishment as gay-related immunodeficiency disease (GRID) for several years, misleading the public into believing that only gay men could be infected with HIV. As a result of stigma and lack of resources, the initial response and research was slow. The virus responsible for the outbreak of illness, HIV, was not identified until 1983.<ref name=HIV_Prevention/> Frustrated with the failure of governments and the medical community to respond adequately to the crisis, hundreds of activist groups formed in the United States and globally to push for better funding for research and treatment. The New York-based group <b>ACT UP</b> was famously vocal in pushing both the US government and drug industry to invest more in developing treatments for HIV/AIDS and changing the way clinical trials are run.<ref name=HIV_Prevention/> | ||
As of 2019, there are an estimate <b>38 million</b> people currently living with HIV world-wide.<ref name=HIV_Infection/><ref name=WHO_HIV/><ref name =HIV_Prevention>[https://www.thelancet.com/action/showPdf?pii=S0140-6736%2808%2960884-3= Merson, M., O’Malley, J., Serwadda, D., Apsuk, C. The history and challenge of HIV prevention. <i>Lancet</i>. 2008. <b>372</b>, 475-488.DOI:10.1016/S0140- 6736(08)60884-3]</ref> Among the HIV-positive population, approximately 81% have received a diagnosis for HIV and 26 million HIV-positive people receive <b>anti-retroviral therapy (ART)</b>.<ref name=WHO_HIV/> Today, the burden of disease is primarily concentrated in sub-Saharan African <b>(Figure 1)</b>.<ref name=Our_World>[https://ourworldindata.org/hiv-aids= Roser, M. and Ritchie, H. “HIV/AIDS,” Our World in Data. November, 2019. https://ourworldindata.org/hiv-aids]</ref> HIV accounts for 1.7% of deaths globally, but HIV mortality varies considerably throughout the world.<ref name=Our_World/> In Europe, HIV accounts for 0.1% of deaths, in the United States it accounts for 0.26% of deaths, and in Brazil it accounts for 1.14% of deaths.<ref name=Our_World/> In South Africa and Botswana, HIV accounts for approximately 28% of deaths, in Kenya it accounts for 17% of deaths, and in Nigeria it accounts for 10.74% of deaths.<ref name=Our_World/> In sub-Saharan African, women account for the majority of new HIV diagnoses, while in the rest of the world, men account for most of the new recorded cases. People under the age of 20 currently account for 2.8 million cases of HIV globally, more than half of whom are under the age of 10.<ref name=Children_HIV>[https://www.nytimes.com/2021/03/31/magazine/pakistan-hiv.html?smid=fb-nytimes&smtyp=cur&fbclid=IwAR2V2M1rF2omhLWvWStR1upUfkfl_81PpM-MLbDolv80ipTDvmwMpp2E6gA= Ouynag, H. and Caron, S. “The City Losing its Children to HIV,” New York Times. March 31, 2021. https://www.nytimes.com/2021/03/31/magazine/pakistan-hiv.html?smid=fb-nytimes&smtyp=cur&fbclid=IwAR2V2M1rF2omhLWvWStR1upUfkfl_81PpM-MLbDolv80ipTDvmwMpp2E6gA]</ref> | |||
In the United States, there are currently about 1.2 million people who are positive for HIV (approximately 14% of whom are unaware of their status).<ref name=US_statistics>[https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics= “U.S Statistics” HIV.gov. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics]</ref> The majority (69%) of new cases occur in men who have sex with men (MSM).<ref name=US_statistics/> It is estimated that about 1 in 8 HIV-positive Americans are unaware of their status.<ref name=ART_Basics>[https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics= “HIV Treatment – The Basics,” HIV.gov. September 24, 2020. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics]</ref> <ref name=PREP>[https://pubmed.ncbi.nlm.nih.gov/29584848/= Riddell, J. Amico, ., Mayer, K. HIV Preexposure Prophylaxis: A Review. <i>JAMA Netowork</i> 2018. 319(12). https://pubmed.ncbi.nlm.nih.gov/29584848/]</ref> HIV disproportionately affects African American and Latinx communities <b>(Figure 3)</b>, accounting for 42% and 27% of new HIV diagnoses, respectively.<ref name=US_statistics/> In 2016, the CDC predicted that one in two Black and one in four Latinx gay and bisexual men would become infected with HIV if current rates of infection continued.<ref name=CDC_predict>[https://www.cdc.gov/nchhstp/newsroom/2016/croi-press-release-risk.html= “Lifetime Risk of HIV Diagnosis,” Centers for Disease Control. February 23, 2016. https://www.cdc.gov/nchhstp/newsroom/2016/croi-press-release-risk.html]</ref> Most cases of HIV in the US today are concentrated in the South, which is home to 21 of the 25 cities with the highest rates of HIV among MSM.<ref name=NYT>[https://www.nytimes.com/2017/06/06/magazine/americas-hidden-hiv-epidemic.html= Linda Vilarosa, “America’s Hidden HIV Epidemic,” New York Times. June 6, 2017. https://www.nytimes.com/2017/06/06/magazine/americas-hidden-hiv-epidemic.html]</ref> Black gay and bisexual men are also less likely to take PrEP, a daily pill which is 99% effective at preventing HIV infection.<ref name=NYT/> | |||
Although HIV remains an enormous challenge to public health, significant improvements have been made in both preventing the spread of HIV and treating the infection. Over the last two decades, the rate new infections have declined by 39% and deaths by 51%, but the overall number of people with HIV has continued to grow <b>(Figure 2)</b>.<ref name=Our_World/> Mortality from HIV/AIDS peaked in 2005 and 2006, when 1.95 million people died annually, but has steadily declined since then.<ref name=Our_World/> Improved outcomes for people with HIV, are due, in large part, to the creation of drugs known as anti-retroviral therapy (ART). ART consists of a combination of three drugs, which each target different components of the HIV life-cycle. Because HIV has such a HIV mutation rate, it is necessary to use a combination of different medications to ensure protection of a patient. Despite tremendous efforts, there still does not exist a vaccine for HIV. Currently, efforts are underway to develop a number of different types of vaccines for HIV. | |||
<br> | |||
<br> | |||
==HIV Life Cycle == | |||
[[Image:life_cycle_HIV.png|thumb|400px|left|<b>Figure 4:</b> Life Cycle of HIV [https://www.nature.com/articles/nrdp201535.pdf].]] | |||
==HIV Life Cycle == | HIV is an RNA <b>retrovirus</b>, which are characterized by their ability to reverse transcribe their own RNA into DNA and then integrate their genome into that of the host.<ref name=HIV_Infection/> The HIV genome consists of two <b>single-stranded, positive-sense mRNAs</b>, with three main open reading frames (<i>gag</i>, <i>pol</i>, and <i>env</i>).<ref name=HIV_Structure>[https://www.nature.com/articles/nrmicro2747.pdf= Engelman, A., Cherepanov, P. The structural biology of HIV-1: mechanistic and therapeutic insights. <i>Nature Reviews Microbiology</i>. 2012. <b>10</b>, 279–290. https://doi.org/10.1038/nrmicro2747]</ref> | ||
<ref name=HIV_Life_Cycle>[https://www.researchgate.net/profile/Frank-Kirchhoff-2/publication/278689737_HIV_Life_Cycle_Overview/links/5760272f08ae2b8d20eb2ee8/HIV-Life-Cycle-Overview.pdf= Kirchhoff, F. HIV Life Cycle. <i>Encyclopedia of AIDS.</i> DOI 10.1007/978-1-4614-9610-6_60-1]</ref> The genome of HIV is also characterized by a high rate of mutation, especially in the genes that encode the envelope protein. <ref name=Spike_Antibodies>[https://www.annualreviews.org/doi/10.1146/annurev-immunol-030409-101256= Mascola, J. and Montefiori, D. The Role of Antibodies in HIV Vaccines. <i>Annual Review of Immunology</i>. 2010. 28, 413-444. https://doi.org/10.1146/annurev-immunol-030409-101256]</ref> The genome is surrounded by nucleocapsid proteins. The viral capsid of HIV includes its genetic material, as well as <b>reverse transcriptase</b>, <b>protease</b>, and <b>integrase</b>.<ref name=HIV_Life_Cycle/> The viral capsid is enclosed by a phospholipid bilayer. The bilayer is embedded with spike proteins, which are responsible for initiating viral entry into a host cell. <b>CD4</b> is the primary receptor targeted by HIV <b>(Figure 4)</b>. CD4 is commonly found on helper T-lymphocytes, macrophages, dendritic cells, and monocytes. | |||
[[Image:spike_protein_structure.png|thumb|250px|right|<b>Figure 5:</b> Negative-stain EM of the HIV spike protein, including gp120 subunits (red), gp41 subunits (green and cyan), and the Fab footprint of neutralizing antibodies. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5226681/pdf/ppat.1006074.pdf].]] | |||
The spike protein is a heterodimer consisting of two subunits: the <b>gp120</b> surface glycoprotein and the <b>gp41</b> transmembrane glycoprotein <b>(Figure 5)</b>.<ref name=Wibmer_2017>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5226681/= Wibmer CK, Gorman J, Ozorowski G, <i>et al</i>. Structure and Recognition of a Novel HIV-1 gp120-gp41 Interface Antibody that Caused MPER Exposure through Viral Escape. <i>PLoS Pathogens</i>. 2017. 3(1):e1006074.. doi:10.1371/journal.ppat.1006074]</ref><ref name=HIV_Structure/> Although this trimer is a well-known target of neutralizing antibodies, the sequence diversity of spike proteins between subtypes of the virus, as well as patients, makes it a challenging target for drugs or vaccines.<ref name=Spike_Antibodies/> | |||
The spike protein mediates cell entry in three steps: <ref name=Wibmer_2017/><ref name=Pancera_2010>[https://pubmed.ncbi.nlm.nih.gov/20080564/= Pancera M, Majeed S, Ban YE, <i>et al</i>. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. <i>Proceedings National Academy of Science USA</i>. 2010.107(3):1166-71. doi: 10.1073/pnas.0911004107.]</ref> | |||
<br> | |||
(1) <b>gp120</b> binds to <b>CD4</b>, inducing a conformational change in the spike protein which allows it to also bind the co-receptor <b>CCR5</b>. | |||
<br> | |||
(2) Binding of gp120 is followed by the insertion of a <b>fusion peptide</b> by <b>gp41</b>. | |||
<br> | |||
(3) The two cell membranes fuse and the virus then enters the host cell. | |||
<br> | |||
Despite the high rate of mutation in the spike protein, broadly neutralizing antibodies can still be produced to bind and act against HIV virions. Multiple epitopes on spike protein have been identified. These are located in the region surrounding the N332 glycan in the external part of gp41 and at the gp41-gp120 interface.<ref name=Wibmer_2017/> <b>Figure 5</b> shows a spike protein generated by negative electron microscopy.<ref name=Wibmer_2017/> The antibody binding sites or “Fab footprints” of the CAP248 antibody are shown within the black dotted circle. Antibodies appear to bind where the spike protein meets the viral membrane. Other studies have also found that the spike protein not only varies in its amino acid sequence, but that it displays conformational diversity. One study found that the gp140 subunit can adopt different post-fusion conformations, which all trigger the insertion of a fusion peptide by gp41.<ref name=Pancera_2010/> These differences in post-fusion conformation allow the spike protein to avoid recognition by the immune system. | |||
Once inside the cell, HIV uses its own <b>reverse transcriptase</b> to convert its mRNA into DNA and then inserts itself into the host genome <b>(Figure 4)</b>. As the virus enters the cytoplasm, it partially uncoats its nucleocapsid protein, but only fully uncoats its extra protein once inside the nucleus. <ref name=HIV_Infection/> <ref name=HIV_Life_Cycle/> The integration of viral DNA into host genome is carried out by the protein <b>integrase</b>. The newly integrated virus can then take over the host cell machinery to direct its own transcription and translation. Although HIV is dependent on many of the proteins provided by the host cell, it also encodes a number of proteins necessary for translation and export in its own genome. For example, HIV encodes <i>Tat</i>, <i>Rev</i>, and <i>Nef</i> proteins. <i>Tat</i>,increases rate of transcription of viral RNA, <i>Rev</i> helps to transport unspliced RNA to cytoplasm, and <i>Nef</i> coordinates the down-regulation of CD4 and MHCI proteins on the cell-surface to evade immune recognition.<ref name=HIV_Life_Cycle/> The initial virions produced are noninfectious precursors. These precursors can be cleaved by <b>protease</b> into their mature and infectious form.<ref name=HIV_Life_Cycle/> Because HIV is a lentivirus, the viral DNA can remain "silently" integrated in the host genome for many years. As a result, many patients do not exhibit symptoms of HIV/AIDS for many years post infection. | |||
[[Image:Anderson.png|thumb|350px|left|<b>Figure 6:</b> Lentiviral Vector which targets CCR5, TRIM5α, and Tat protein reduces the quantity of p24 antigen (HIV virions) as compared with nontransduced and positive control (CCL-c EGFP) cells. [http://web.b.ebscohost.com.libproxy.kenyon.edu/ehost/pdfviewer/pdfviewer?vid=5&sid=32260430-fa32-4ff2-8245-90bc102e5091%40sessionmgr103].]] | |||
Understanding each step of the HIV life cycle has been crucial in developing drugs and that target HIV. In <b>Figure 4</b>, each of the red boxes represents a step in the HIV life cycle which is targeted by an existing drug. In one study, researchers developed a lentiviral vector that contained three main components: a short-hairpin to knockdown CCR5 expression, a “TAR decoy” binds the Tat protein in order to prevent transcription, and a TRIM5α molecule to evade detection from the immune system.<ref name=Anderson>[http://web.b.ebscohost.com.libproxy.kenyon.edu/ehost/pdfviewer/pdfviewer?vid=5&sid=32260430-fa32-4ff2-8245-90bc102e5091%40sessionmgr103= Anderson, J., Javien, J., Nolta, J., Bauer, G. Preintegration HIV Inhibition by a Combination Lentiviral Vector Containing a Chimeric TRIM5a Protein, a CCR5 shRNA, and a TAR Decoy. <i>American Society of Gene and Cell Therapy</i>. 2009. 17(12). http://europepmc.org/article/PMC/2814390]</ref> Using a p24 antigen enzyme immunoassay, they measured the level of the viral capsid protein p24 as a proxy for the quantity of viral particles present. The researchers found that, compared to the nontransduced cells and EGFP controls, the cells that were transduced with a combination vector had greatly reduced levels of HIV virus <b>(Figure 6)</b>. While the amount of p24 seemed to climb rapidly in negative (Nontransduced) and positive (CCL-c EGFP) controls, the quantity of p24 detected in transducer cells (CCL-c combination) increased only slightly.<ref name=Anderson/> These results suggest that blocking multiple steps of the HIV Life Cycle, such as binding the co-receptor CCR5, transcription, and evasion of the immune system, could be an effective tool for drugs and therapies. This study, however, was done <i>in vitro</i> and does not necessarily prove such a vector would be effective in a clinical setting. | |||
<br> | |||
<br> | |||
<br> | <br> | ||
== | ==Antiretroviral Therapy (ART)== | ||
The development of <b>antiretroviral drugs (ART)</b> represents a major breakthrough in the effort to tackle HIV. ART prevents the virus from replicating inside a patient, reducing the <b>viral load</b> or amount of virus present inside a patient’s body.<ref name=ART_Basics>[https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics= “HIV Treatment – The Basics,” HIV.gov. September 24, 2020. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics]</ref>Although ART cannot eliminate the virus in infected individuals, it is largely responsible for the great reductions in mortality and morbidity caused by HIV/AIDS. As of 2017, there were approximately 17 million people taking ART around the globe.<ref name=Developing_an_HIV_Vaccine>[https://science.sciencemag.org/content/sci/355/6330/1129.full.pdf= Haynes, B. and Burton, D. Developing an HIV Vaccine. <i>Science</i>. 2017. 355(6330). DOI: 10.1126/science.aan0662]</ref> Patients who take ART have higher CD4 T-cell counts, making them less vulnerable to other infections. A reduction in viral load is also associated with a reduced risk for transmission.<ref name=ART_Basics/> As a result, ART is a useful tool for both improving health outcomes in individual patients, as well as in reducing the incidence of HIV in the population. There currently exist six classes of antiretroviral drugs used to treat individuals with HIV: <ref name=Annual_Reviews_HIV_Drugs>[https://www.annualreviews.org/doi/pdf/10.1146/annurev-med-041217-013717= Gulick, R. and Flexner, C. Long-Acting HIV Drugs for Treatment and Prevention. 2019. <i>Annual Reviews of Medicine</i>. <b>70</b>, 137-50. https://doi.org/10.1146/annurev- med- 041217- 013717]</ref><ref name=Current_Treatment>[https://reader.elsevier.com/reader/sd/pii/S1879625716300207?token=CF3A2EB384772DB8DE36875E0884379AADA948ECA270856F5CB0A53AC5D222DCA42E5A0220E4AE88971FDE5BC3CA31A8&originRegion=us-east-1&originCreation=20210407002244= Cihlar, T. and Fordyce, M. Current Status and Prospects of HIV Treatment. 2016. <i>Current Opinion in Virology</i>. <b>18</b>, 50-56.]</ref> | |||
<br> | <br> | ||
:(1) Nucleoside Reverse Transcriptase Inhibitors | |||
:(2) Nonnucleoside Reverse Transcriptase Inhibitors | |||
:(3) Protease Inhibitors | |||
:(4) Integrase Inhibitors | |||
:(5) Entry Inhibitors | |||
:(6) Capsid Inhibitors | |||
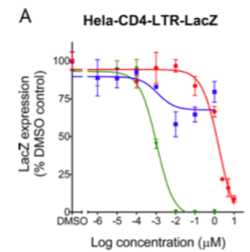
[[Image:Ebselen.png|thumb|250px|right|<b>Figure 6:</b> LacZ as a measure of the number of HIV-infected CD4 cells exposed to varying concentrations of Efavirenez (green), Lopinavir (blue), and Ebselen (red). [https://pubmed.ncbi.nlm.nih.gov/26810656/].]] | |||
The first antiretroviral drug for HIV, zidovudine <b>(AZT)</b>, which was created in 1987, is a <b>nucleoside reverse transcriptase inhibitor (NRTI)</b>.<ref name=AZT>[https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0031558= Brehm, J., Scott, Y., Koontz, D., Perry, S. et al. Zidovudine (AZT) Monotherapy Selects for the A360V Mutation in the Connection Domain of HIV-1 Reverse Transcriptase. 2012. 7(2). https://doi.org/10.131/journal.pone.0031558]</ref> AZT acts as a thymidine analogue and is incorporated into the DNA by reverse transcriptase. AZT, however, lacks a 3’ -OH. Incorporation of the drug then results in termination of DNA synthesis. Without a completed DNA strand, the virus cannot be integrated into the host genome. The first cases of AZT resistance were documented in 1989.<ref name=AZT/> AZT-resistant strains of HIV have been characterized by the existence of mutations in the polymerase domain and connection domain of their reverse transcriptase. AZT is no longer widely used in many countries both because of resistance and side effects. Side effects of AZT include anaemia, hepatic steatosis, lactic acidosis, and lipatrophy.<ref name=HIV_Infection/> Most AZT is prescribed in low- and middle-income countries. Other NRTIs, such as tenofovir and emtricitabine, also act as nucleotide analogues to block the synthesis of dsDNA.<ref name=HIV_Infection/> | |||
<b>Nonnucleoside Reverse Transcriptase Inhibitors (NNRTI)</b> also block the activity of reverse transcriptase, but through a different mechanism than NRTIs. These drugs bind to and block the active site of reverse transcriptase, preventing it from synthesizing dsDNA from viral RNA.<ref name=HIV_Infection/> NNRTIs are considered to be both safe and effective, although some are associated with an increased risk of depression and other neuropsychiatric reactions.<ref name=HIV_Infection/><ref name=NNRTI>[https://academic.oup.com/jac/article/70/10/2693/830680?login=true=Apostolova, N., Funes, H., Blas-Garcia, A., Galindo, M. <i>et al.</i>. Efavirenz and the CNS: what we already know and questions that need to be answered. <i>Journal of Antimicrobial Chemotherapy</i>. 70(10) 2693–2708, https://doi.org/10.1093/jac/dkv183]</ref> Efavirenz is an NNRTI that is widely-used in many high-income countries.<ref name=HIV_Infection/> | |||
<b>Protease inhibitors</b> block the enzyme protease from cleaving immature virions into mature, infectious progeny.<ref name=HIV_Infection/> <b>Integrase inhibitors</b> block integration of viral DNA into host genome. <b>Entry inhibitors</b> block entry into the host cell. One entry inhibitor, maraviroc, binds CCR5 to block the gp120 protein from binding the co-receptor. <b>Capsid inhibitors</b> represent a more recent class of antiretroviral drugs. A recently developed capsid inhibitor, Ebselen, binds to the C-terminal domain and blocks dimerization. One study found that Ebselen inhibits HIV-1 replication. Using a cell line that stably expresses B-galactosidase (LacZ), researchers found that exposure to higher concentrations of Ebselen lead to reduced numbers of HIV infected Hela-CD4-LTR-LacZ cells <b>(Figure 6)</b>. In other words, the fewer HIV-infected LacZ expressing cells, the less B-galactosidase was measured. <ref name=Ebselen>[https://aac.asm.org/content/aac/60/4/2195.full.pdf= Thenin-Houssier S, de Vera IM, Pedro-Rosa L, Brady A, Richard A, Konnick B, Opp S, Buffone C, Fuhrmann J, Kota S, <i>et al</i>. Ebselen, a Small-Molecule Capsid Inhibitor of HIV-1 Replication. <i>Antimicrob Agents Chemother.</i> 2016. 60(4). doi: 10.1128/AAC.02574-15.]</ref> | |||
Because of the high rate of mutation in HIV, individuals now receive <b>combination therapy</b> or a combination of three of the above antiretroviral drugs.<ref name=HIV_Infection/> | |||
== | ==Vaccines: B-Cell Immunogens== | ||
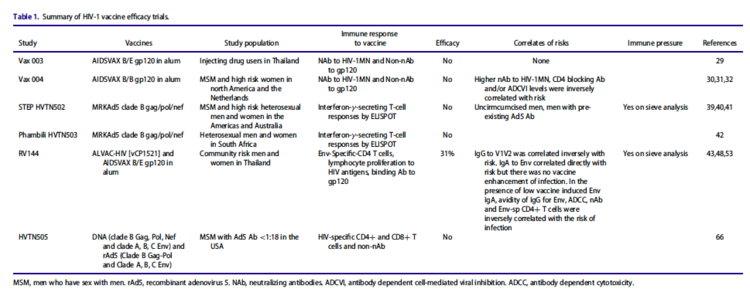
Although there currently does not exist a vaccine for HIV, there are numerous vaccine candidates in development in Phase I-III clinical trials. Because ART is costly, difficult to distribute, and not entirely effective at eliminating infection or preventing transmission, there is enormous need to develop a universal HIV vaccine. Between 1987 and 2013, five out of six HIV vaccine efficacy trials have been unsuccessful, with only one showing low efficacy.<ref name=Developing_Vaccine>[https://science.sciencemag.org/content/sci/355/6330/1129.full.pdf= Haynes, B. and Burton, D. Developing an HIV Vaccine. <i>Science</i>. 2017. 355(6330). DOI: 10.1126/science.aan0662]</ref> There are two main strategies for vaccine development that are currently being pursued: (1) B-cell immunogens, which would stimulate production of broadly neutralizing antibodies, and (2) T-cell immunogens, which stimulate production of antiviral T-cells.<ref name=IAVI>[https://www.iavi.org/our-science/hiv-vaccines= “HIV Vaccines,” Iavi. https://www.iavi.org/our-science/hiv-vaccines]</ref> | |||
[[Image:Vaccine_Trials.png|thumb|750px|center|<b>Figure 7:</b> Overview of six major HIV vaccine efficacy trials from 1987-2013. [https://www.tandfonline.com/doi/pdf/10.1080/21645515.2016.1276138?needAccess=true].]] | |||
<br> | <br> | ||
[[Image:Neutralization_Potency.png|thumb|250px|right|<b>Figure 8:</b> The Neutralization Breadth of BnABs is correlated with their serum potency. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4042313/pdf/nihms585901.pdf].]] | |||
<b>Broadly neutralizing antibodies (BnAb</b>) bind to a pathogen and prevent it from carrying out its function. They are also to bind a diverse range of pathogens with sequence variation.<ref name=Developing_Vaccine/><ref name=Vaccine_Progress>[https://www.tandfonline.com/doi/pdf/10.1080/21645515.2016.1276138?needAccess=true= Hsu, D. and O’Connell, R. Progress in HIV Vaccine Development. <i>Taylor and Francis Group</i>. 2017. 13(5). https://doi.org/10.1080/21645515.2016.1276138]</ref> Because of their efficacy and ability to bind diverse pathogens, broadly neutralizing antibodies are a compelling tool for eliciting an immune response against HIV. BnAbs primarily target Env proteins on HIV. Approximately 20-30% of HIV-positive individuals BnAbs capable of targeting a wide-range of viral subtypes.<ref name=Vaccine_Progress/> BnAbs have also been shown to protect against the transmission of retroviruses in monkeys exposed to simian-human chimeric immunodeficiency viruses (SHIV).<ref name=Developing_Vaccines/> There are many challenges associated with stimulating a sufficient BnAb response. The antibodies have to be able to access the right epitope on the HIV spike protein, much of which is blocked by glycosylated proteins. The BnAbs also have to be “cross-reactive” or able to bind a diverse array of spike proteins. Most efforts to develop a vaccine that elicits a broadly neutralizing antibody response have been unsuccessful in eliciting a sufficient antibody response to prevent infection. One study, which looked at BnAbs that were produced in HIV-positive individuals in natural infection, found that the neutralization breadth of antibodies was highly correlated with their potency.<ref name=Neutralizing_Antibody_Response>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4042313/= Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. <i>AIDS</i>. 2014;28(2) doi:10.1097/QAD.0000000000000106]</ref> In other words, the more diverse the array of gp41/gp120 epitopes the BnAbs could bind, the more effective they were at binding <b>(Figure 8)</b>. This suggests that a vaccine that elicits cross-reactive BnAbs may be effective in preventing infection. They also found that only a small number (10%) of naturally-occurring BnAbs are broadly cross-reactive. | |||
== | Researchers at the Scripps Research Institute developed a vaccine that induces a broadly neutralizing antibody response which recently passed Phase I of the clinical trial, which tested the vaccine in human participants. This vaccine is able to elicit a broadly neutralizing antibody response by targeting a specific subset of naïve B-cells. Naive B-cells are useful because they have not undergone the degree of mutation that other B-cells have.<ref name=Scripps_Vaccine>[https://www.nature.com/articles/s41467-020-19650-8= Huang, D., Tran, J.T., Olson, A. et al. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. <i>Nat Commun</i> <b>11</b>, 5850 (2020). https://doi.org/10.1038/s41467-020-19650-8]</ref> | ||
==Vaccines: Viral Vectors== | |||
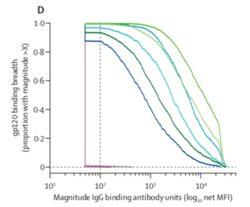
[[Image:lancet.png|thumb|250px|left|<b>Figure 9:</b> Comparison of trivalent mosaic vaccine (blue), tetravalent mosaic vaccine (green) and placebo group (purple) gp120 binding and IgG antibody. | |||
[https://www.thelancet.com/action/showPdf?pii=S2352-3018%2820%2930229-0].]] | |||
Vectors are a useful tool for delivering vaccines into target cells. Several efforts have been made to use a vector model to develop a vaccine for HIV. Vectors are other viruses which themselves do not cause disease.<ref name=Types_of_Vaccines?>[https://www.hvtn.org/en/science/hiv-vaccine-basics/types-vaccines.html= HIV Trials Network, “Types of Vaccines,” Fred Hutchinson. https://www.hvtn.org/en/science/hiv-vaccine-basics/types-vaccines.html]</ref> DNA is inserted into the vector which encodes the proteins necessary to elicit an immune response. Currently, several vectors, including Ad26, Ad35, Ad4, and CMV, are being developed for possible use against HIV. | |||
Of the six clinical trials run for HIV vaccines, <b>RV144</b> was the only one to demonstrate a vaccine had some efficacy (60% at 60 months and 31% at 3.5 years) <b>(Figure 9)</b>.<ref name=Tand_HIV_Vaccine>[https://www.tandfonline.com/doi/pdf/10.1080/21645515.2016.1276138?needAccess=true= Hsu, D. and O’Connell, R. Progress in HIV Vaccine Development. <i>Taylor and Francis Group</i>. 2017. 13(5). https://doi.org/10.1080/21645515.2016.1276138]</ref> RV144 was a nonreplicating viral vector vaccine which used a recombinant canarypox vector to deliver <i>Gag</i>, <i>Env</i>, and <i>Pro</i> immunogens into target cells.<ref name=Tand_HIV_Vaccine/><ref name=Parks>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4040527/= Parks, C., Picker, L., King, C. Development of replication-competent viral vectors for HIV vaccine delivery. <i>Curr Opin HIV AIDS</i>. 2013;8(5) doi:10.1097/COH.0b013e328363d389]</ref>Although this study showed considerable promise for vector-based vaccines, not all studies have been as successful. In another clinical trial, known as STEP, patients who received a vaccine with an Ad5 vector were not protected against HIV and had an increased incidence of acquiring the infection.<ref name=Tand_HIV_Vaccine/> | |||
Recent efforts have been focused on creating <b>mosaic vaccines</b>, which aim to stimulate an immune response against a wider variety of HIV strains.<ref name=HIV_Infection/> A new vaccine, which uses the <b>Ad26 vector</b> to deliver an HIV antigen is currently under development. A 2020 study from <i>The Lancet</i> found that individuals who received a tetrameric Ad25 mosaic vaccine had higher IgG titres, as well as greater magnitude and breadth of binding Env proteins, than those who received a trimeric Ad25 mosaic vaccine and the placebo group.<ref name=Lancet>[https://www.thelancet.com/action/showPdf?pii=S2352-3018%2820%2930229-0= Baden, L, Stieh, D., Sarnecki, M., et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. <i> Lancet</i> 2020. 7(10). https://doi.org/10.1016/S2352-3018(20)30229-0]</ref> | |||
==Conclusion== | ==Conclusion== | ||
Since the first reported cases of HIV in 1981, HIV/AIDS has become one of the greatest challenges to public health around the world. HIV poses several unique challenges for the development of drugs to treat infection and vaccines to prevent infection | |||
:(1) HIV is integrated into the host genome, where it can lie dormant for many years. Unless the virus has started producing mRNA and protein products, it can be difficult to detect infected cells during the latency period. | |||
:(2) HIV is extremely skilled at evading the immune system. | |||
:(3) The envelope of the virion is heavily glycosylated, making it difficult to target with antibodies. | |||
:(4) HIV has a very high mutation rate. Its genetic sequence can vary both between and within patients. There is a particularly high degree of sequence diversity in envelope genes (encoded by the env open reading frame). | |||
Great advancements have been made in the development of antiretroviral drugs, which can reduce morbidity and likelihood of transmission, but cannot eliminate the virus from individuals. Antiretroviral drugs have saved millions of lives around the world, but they are costly to manufacture, difficult to distribute, and often require individuals to take medication on a daily basis. It has become urgently necessary to develop a universal vaccine to protect individuals from infection with HIV, but the unique properties of the virus have made this effort distinctly challenging. HIV has a high mutation rate, particularly in its envelope proteins, it can hide within the genome, it is highly adept at evading the mechanisms of the immune system, and its envelope proteins are heavily glycosylated, hiding it from the effects of neutralizing antibodies. Nonetheless, significant progress has been made and there are numerous vaccine candidates currently in development. | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Latest revision as of 18:49, 13 April 2021
Introduction
By Alice Tillman
Human immunodeficiency virus (HIV) is an RNA retrovirus which attacks the immune system of the infected individual. There are two main strains of HIV: HIV-1 and HIV-2. HIV-1 is the more prevalent and pathogenic of the two strains and therefore is the subject of most research, including this Wiki Page. Genetic sequencing techniques have revealed that HIV-1 and HIV-2 originated from separate cross-over transmission events from simian immunodeficiency virus (SIV) in chimpanzees and sooty mangabeys.[1] HIV belongs to a type of retroviruses which are called lentiviruses. Lentiviruses infect their hosts over a very long period of time and it can take years for symptoms to manifest. Over time, HIV can progress into Acquired Immunodeficiency Syndrome or AIDS. HIV/AIDS is characterized by a decline in the number of CD4+ T-cells and an increased susceptibility to other infections.[2]
HIV is transmitted from one person to another through bodily fluids, such as blood, semen, vaginal fluid, and breast milk.[3][4] In order for an infection to take place, the virus must reach the bloodstream of an individual. Transmission most commonly occurs during vaginal or anal sex, through injection with needles, or from mother to child during pregnancy, labor, or breastfeeding.[2][5][4] A mother with HIV has a 15-45% chance of transmitting the virus to her child.[6] HIV-positive women who take anti-viral medication during pregnancy and 4-6 weeks after childbirth have only a 5% chance of passing on the virus.[6]
The earliest cases of HIV were documented on June 5, 1981, when the CDC noted that five men in Los Angeles had developed pneumonia from the fungus Pneumocystis jirovecii in the Morbidity and Mortality Weekly Report.[7] In the first several years of the pandemic, HIV/AIDS was mostly recorded in predominately white, gay communities in urban areas.[2][7] The disease caused by HIV, AIDS, was referred to in the medical establishment as gay-related immunodeficiency disease (GRID) for several years, misleading the public into believing that only gay men could be infected with HIV. As a result of stigma and lack of resources, the initial response and research was slow. The virus responsible for the outbreak of illness, HIV, was not identified until 1983.[7] Frustrated with the failure of governments and the medical community to respond adequately to the crisis, hundreds of activist groups formed in the United States and globally to push for better funding for research and treatment. The New York-based group ACT UP was famously vocal in pushing both the US government and drug industry to invest more in developing treatments for HIV/AIDS and changing the way clinical trials are run.[7]
As of 2019, there are an estimate 38 million people currently living with HIV world-wide.[2][5][7] Among the HIV-positive population, approximately 81% have received a diagnosis for HIV and 26 million HIV-positive people receive anti-retroviral therapy (ART).[5] Today, the burden of disease is primarily concentrated in sub-Saharan African (Figure 1).[8] HIV accounts for 1.7% of deaths globally, but HIV mortality varies considerably throughout the world.[8] In Europe, HIV accounts for 0.1% of deaths, in the United States it accounts for 0.26% of deaths, and in Brazil it accounts for 1.14% of deaths.[8] In South Africa and Botswana, HIV accounts for approximately 28% of deaths, in Kenya it accounts for 17% of deaths, and in Nigeria it accounts for 10.74% of deaths.[8] In sub-Saharan African, women account for the majority of new HIV diagnoses, while in the rest of the world, men account for most of the new recorded cases. People under the age of 20 currently account for 2.8 million cases of HIV globally, more than half of whom are under the age of 10.[9]
In the United States, there are currently about 1.2 million people who are positive for HIV (approximately 14% of whom are unaware of their status).[10] The majority (69%) of new cases occur in men who have sex with men (MSM).[10] It is estimated that about 1 in 8 HIV-positive Americans are unaware of their status.[11] [12] HIV disproportionately affects African American and Latinx communities (Figure 3), accounting for 42% and 27% of new HIV diagnoses, respectively.[10] In 2016, the CDC predicted that one in two Black and one in four Latinx gay and bisexual men would become infected with HIV if current rates of infection continued.[13] Most cases of HIV in the US today are concentrated in the South, which is home to 21 of the 25 cities with the highest rates of HIV among MSM.[14] Black gay and bisexual men are also less likely to take PrEP, a daily pill which is 99% effective at preventing HIV infection.[14]
Although HIV remains an enormous challenge to public health, significant improvements have been made in both preventing the spread of HIV and treating the infection. Over the last two decades, the rate new infections have declined by 39% and deaths by 51%, but the overall number of people with HIV has continued to grow (Figure 2).[8] Mortality from HIV/AIDS peaked in 2005 and 2006, when 1.95 million people died annually, but has steadily declined since then.[8] Improved outcomes for people with HIV, are due, in large part, to the creation of drugs known as anti-retroviral therapy (ART). ART consists of a combination of three drugs, which each target different components of the HIV life-cycle. Because HIV has such a HIV mutation rate, it is necessary to use a combination of different medications to ensure protection of a patient. Despite tremendous efforts, there still does not exist a vaccine for HIV. Currently, efforts are underway to develop a number of different types of vaccines for HIV.
HIV Life Cycle

HIV is an RNA retrovirus, which are characterized by their ability to reverse transcribe their own RNA into DNA and then integrate their genome into that of the host.[2] The HIV genome consists of two single-stranded, positive-sense mRNAs, with three main open reading frames (gag, pol, and env).[15] [16] The genome of HIV is also characterized by a high rate of mutation, especially in the genes that encode the envelope protein. [17] The genome is surrounded by nucleocapsid proteins. The viral capsid of HIV includes its genetic material, as well as reverse transcriptase, protease, and integrase.[16] The viral capsid is enclosed by a phospholipid bilayer. The bilayer is embedded with spike proteins, which are responsible for initiating viral entry into a host cell. CD4 is the primary receptor targeted by HIV (Figure 4). CD4 is commonly found on helper T-lymphocytes, macrophages, dendritic cells, and monocytes.
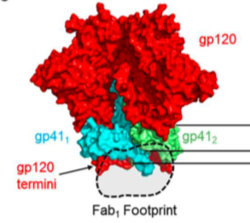
The spike protein is a heterodimer consisting of two subunits: the gp120 surface glycoprotein and the gp41 transmembrane glycoprotein (Figure 5).[18][15] Although this trimer is a well-known target of neutralizing antibodies, the sequence diversity of spike proteins between subtypes of the virus, as well as patients, makes it a challenging target for drugs or vaccines.[17]
The spike protein mediates cell entry in three steps: [18][19]
(1) gp120 binds to CD4, inducing a conformational change in the spike protein which allows it to also bind the co-receptor CCR5.
(2) Binding of gp120 is followed by the insertion of a fusion peptide by gp41.
(3) The two cell membranes fuse and the virus then enters the host cell.
Despite the high rate of mutation in the spike protein, broadly neutralizing antibodies can still be produced to bind and act against HIV virions. Multiple epitopes on spike protein have been identified. These are located in the region surrounding the N332 glycan in the external part of gp41 and at the gp41-gp120 interface.[18] Figure 5 shows a spike protein generated by negative electron microscopy.[18] The antibody binding sites or “Fab footprints” of the CAP248 antibody are shown within the black dotted circle. Antibodies appear to bind where the spike protein meets the viral membrane. Other studies have also found that the spike protein not only varies in its amino acid sequence, but that it displays conformational diversity. One study found that the gp140 subunit can adopt different post-fusion conformations, which all trigger the insertion of a fusion peptide by gp41.[19] These differences in post-fusion conformation allow the spike protein to avoid recognition by the immune system.
Once inside the cell, HIV uses its own reverse transcriptase to convert its mRNA into DNA and then inserts itself into the host genome (Figure 4). As the virus enters the cytoplasm, it partially uncoats its nucleocapsid protein, but only fully uncoats its extra protein once inside the nucleus. [2] [16] The integration of viral DNA into host genome is carried out by the protein integrase. The newly integrated virus can then take over the host cell machinery to direct its own transcription and translation. Although HIV is dependent on many of the proteins provided by the host cell, it also encodes a number of proteins necessary for translation and export in its own genome. For example, HIV encodes Tat, Rev, and Nef proteins. Tat,increases rate of transcription of viral RNA, Rev helps to transport unspliced RNA to cytoplasm, and Nef coordinates the down-regulation of CD4 and MHCI proteins on the cell-surface to evade immune recognition.[16] The initial virions produced are noninfectious precursors. These precursors can be cleaved by protease into their mature and infectious form.[16] Because HIV is a lentivirus, the viral DNA can remain "silently" integrated in the host genome for many years. As a result, many patients do not exhibit symptoms of HIV/AIDS for many years post infection.
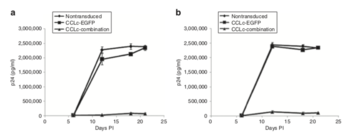
Understanding each step of the HIV life cycle has been crucial in developing drugs and that target HIV. In Figure 4, each of the red boxes represents a step in the HIV life cycle which is targeted by an existing drug. In one study, researchers developed a lentiviral vector that contained three main components: a short-hairpin to knockdown CCR5 expression, a “TAR decoy” binds the Tat protein in order to prevent transcription, and a TRIM5α molecule to evade detection from the immune system.[20] Using a p24 antigen enzyme immunoassay, they measured the level of the viral capsid protein p24 as a proxy for the quantity of viral particles present. The researchers found that, compared to the nontransduced cells and EGFP controls, the cells that were transduced with a combination vector had greatly reduced levels of HIV virus (Figure 6). While the amount of p24 seemed to climb rapidly in negative (Nontransduced) and positive (CCL-c EGFP) controls, the quantity of p24 detected in transducer cells (CCL-c combination) increased only slightly.[20] These results suggest that blocking multiple steps of the HIV Life Cycle, such as binding the co-receptor CCR5, transcription, and evasion of the immune system, could be an effective tool for drugs and therapies. This study, however, was done in vitro and does not necessarily prove such a vector would be effective in a clinical setting.
Antiretroviral Therapy (ART)
The development of antiretroviral drugs (ART) represents a major breakthrough in the effort to tackle HIV. ART prevents the virus from replicating inside a patient, reducing the viral load or amount of virus present inside a patient’s body.[11]Although ART cannot eliminate the virus in infected individuals, it is largely responsible for the great reductions in mortality and morbidity caused by HIV/AIDS. As of 2017, there were approximately 17 million people taking ART around the globe.[21] Patients who take ART have higher CD4 T-cell counts, making them less vulnerable to other infections. A reduction in viral load is also associated with a reduced risk for transmission.[11] As a result, ART is a useful tool for both improving health outcomes in individual patients, as well as in reducing the incidence of HIV in the population. There currently exist six classes of antiretroviral drugs used to treat individuals with HIV: [22][23]
- (1) Nucleoside Reverse Transcriptase Inhibitors
- (2) Nonnucleoside Reverse Transcriptase Inhibitors
- (3) Protease Inhibitors
- (4) Integrase Inhibitors
- (5) Entry Inhibitors
- (6) Capsid Inhibitors
The first antiretroviral drug for HIV, zidovudine (AZT), which was created in 1987, is a nucleoside reverse transcriptase inhibitor (NRTI).[24] AZT acts as a thymidine analogue and is incorporated into the DNA by reverse transcriptase. AZT, however, lacks a 3’ -OH. Incorporation of the drug then results in termination of DNA synthesis. Without a completed DNA strand, the virus cannot be integrated into the host genome. The first cases of AZT resistance were documented in 1989.[24] AZT-resistant strains of HIV have been characterized by the existence of mutations in the polymerase domain and connection domain of their reverse transcriptase. AZT is no longer widely used in many countries both because of resistance and side effects. Side effects of AZT include anaemia, hepatic steatosis, lactic acidosis, and lipatrophy.[2] Most AZT is prescribed in low- and middle-income countries. Other NRTIs, such as tenofovir and emtricitabine, also act as nucleotide analogues to block the synthesis of dsDNA.[2]
Nonnucleoside Reverse Transcriptase Inhibitors (NNRTI) also block the activity of reverse transcriptase, but through a different mechanism than NRTIs. These drugs bind to and block the active site of reverse transcriptase, preventing it from synthesizing dsDNA from viral RNA.[2] NNRTIs are considered to be both safe and effective, although some are associated with an increased risk of depression and other neuropsychiatric reactions.[2][25] Efavirenz is an NNRTI that is widely-used in many high-income countries.[2]
Protease inhibitors block the enzyme protease from cleaving immature virions into mature, infectious progeny.[2] Integrase inhibitors block integration of viral DNA into host genome. Entry inhibitors block entry into the host cell. One entry inhibitor, maraviroc, binds CCR5 to block the gp120 protein from binding the co-receptor. Capsid inhibitors represent a more recent class of antiretroviral drugs. A recently developed capsid inhibitor, Ebselen, binds to the C-terminal domain and blocks dimerization. One study found that Ebselen inhibits HIV-1 replication. Using a cell line that stably expresses B-galactosidase (LacZ), researchers found that exposure to higher concentrations of Ebselen lead to reduced numbers of HIV infected Hela-CD4-LTR-LacZ cells (Figure 6). In other words, the fewer HIV-infected LacZ expressing cells, the less B-galactosidase was measured. [26]
Because of the high rate of mutation in HIV, individuals now receive combination therapy or a combination of three of the above antiretroviral drugs.[2]
Vaccines: B-Cell Immunogens
Although there currently does not exist a vaccine for HIV, there are numerous vaccine candidates in development in Phase I-III clinical trials. Because ART is costly, difficult to distribute, and not entirely effective at eliminating infection or preventing transmission, there is enormous need to develop a universal HIV vaccine. Between 1987 and 2013, five out of six HIV vaccine efficacy trials have been unsuccessful, with only one showing low efficacy.[27] There are two main strategies for vaccine development that are currently being pursued: (1) B-cell immunogens, which would stimulate production of broadly neutralizing antibodies, and (2) T-cell immunogens, which stimulate production of antiviral T-cells.[28]
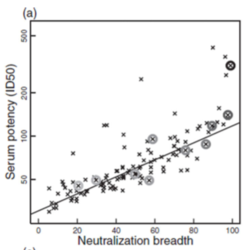
Broadly neutralizing antibodies (BnAb) bind to a pathogen and prevent it from carrying out its function. They are also to bind a diverse range of pathogens with sequence variation.[27][29] Because of their efficacy and ability to bind diverse pathogens, broadly neutralizing antibodies are a compelling tool for eliciting an immune response against HIV. BnAbs primarily target Env proteins on HIV. Approximately 20-30% of HIV-positive individuals BnAbs capable of targeting a wide-range of viral subtypes.[29] BnAbs have also been shown to protect against the transmission of retroviruses in monkeys exposed to simian-human chimeric immunodeficiency viruses (SHIV).[30] There are many challenges associated with stimulating a sufficient BnAb response. The antibodies have to be able to access the right epitope on the HIV spike protein, much of which is blocked by glycosylated proteins. The BnAbs also have to be “cross-reactive” or able to bind a diverse array of spike proteins. Most efforts to develop a vaccine that elicits a broadly neutralizing antibody response have been unsuccessful in eliciting a sufficient antibody response to prevent infection. One study, which looked at BnAbs that were produced in HIV-positive individuals in natural infection, found that the neutralization breadth of antibodies was highly correlated with their potency.[31] In other words, the more diverse the array of gp41/gp120 epitopes the BnAbs could bind, the more effective they were at binding (Figure 8). This suggests that a vaccine that elicits cross-reactive BnAbs may be effective in preventing infection. They also found that only a small number (10%) of naturally-occurring BnAbs are broadly cross-reactive.
Researchers at the Scripps Research Institute developed a vaccine that induces a broadly neutralizing antibody response which recently passed Phase I of the clinical trial, which tested the vaccine in human participants. This vaccine is able to elicit a broadly neutralizing antibody response by targeting a specific subset of naïve B-cells. Naive B-cells are useful because they have not undergone the degree of mutation that other B-cells have.[32]
Vaccines: Viral Vectors
Vectors are a useful tool for delivering vaccines into target cells. Several efforts have been made to use a vector model to develop a vaccine for HIV. Vectors are other viruses which themselves do not cause disease.[33] DNA is inserted into the vector which encodes the proteins necessary to elicit an immune response. Currently, several vectors, including Ad26, Ad35, Ad4, and CMV, are being developed for possible use against HIV.
Of the six clinical trials run for HIV vaccines, RV144 was the only one to demonstrate a vaccine had some efficacy (60% at 60 months and 31% at 3.5 years) (Figure 9).[34] RV144 was a nonreplicating viral vector vaccine which used a recombinant canarypox vector to deliver Gag, Env, and Pro immunogens into target cells.[34][35]Although this study showed considerable promise for vector-based vaccines, not all studies have been as successful. In another clinical trial, known as STEP, patients who received a vaccine with an Ad5 vector were not protected against HIV and had an increased incidence of acquiring the infection.[34]
Recent efforts have been focused on creating mosaic vaccines, which aim to stimulate an immune response against a wider variety of HIV strains.[2] A new vaccine, which uses the Ad26 vector to deliver an HIV antigen is currently under development. A 2020 study from The Lancet found that individuals who received a tetrameric Ad25 mosaic vaccine had higher IgG titres, as well as greater magnitude and breadth of binding Env proteins, than those who received a trimeric Ad25 mosaic vaccine and the placebo group.[36]
Conclusion
Since the first reported cases of HIV in 1981, HIV/AIDS has become one of the greatest challenges to public health around the world. HIV poses several unique challenges for the development of drugs to treat infection and vaccines to prevent infection
- (1) HIV is integrated into the host genome, where it can lie dormant for many years. Unless the virus has started producing mRNA and protein products, it can be difficult to detect infected cells during the latency period.
- (2) HIV is extremely skilled at evading the immune system.
- (3) The envelope of the virion is heavily glycosylated, making it difficult to target with antibodies.
- (4) HIV has a very high mutation rate. Its genetic sequence can vary both between and within patients. There is a particularly high degree of sequence diversity in envelope genes (encoded by the env open reading frame).
Great advancements have been made in the development of antiretroviral drugs, which can reduce morbidity and likelihood of transmission, but cannot eliminate the virus from individuals. Antiretroviral drugs have saved millions of lives around the world, but they are costly to manufacture, difficult to distribute, and often require individuals to take medication on a daily basis. It has become urgently necessary to develop a universal vaccine to protect individuals from infection with HIV, but the unique properties of the virus have made this effort distinctly challenging. HIV has a high mutation rate, particularly in its envelope proteins, it can hide within the genome, it is highly adept at evading the mechanisms of the immune system, and its envelope proteins are heavily glycosylated, hiding it from the effects of neutralizing antibodies. Nonetheless, significant progress has been made and there are numerous vaccine candidates currently in development.
References
- ↑ Lemey, P., Pybus, O., Wang, B., Saksena, N., Salemi, M., Vandamme, A. Tracing the origin and history of the HIV-2 epidemic. Proceedings of National Academy Science USA. 2003, 100(11):6588-6592. doi:10.1073/pnas.0936469100
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Deeks, S., Overbaugh, J., Phillips, A., et al. HIV infection. Nature Reviews Disease Primers. 2015. 1, 15035. https://doi.org/10.1038/nrdp.2015.35
- ↑ “How is HIV Transmitted?” HIV.gov https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/how-is-hiv-transmitted
- ↑ 4.0 4.1 “HIV and AIDS – basic facts,” UNAIDS. https://www.unaids.org/en/frequently-asked-questions-about-hiv-and-aids
- ↑ 5.0 5.1 5.2 “HIV/AIDS,” World Health Organization. November 30, 2020. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- ↑ 6.0 6.1 “Mother-to-child transmission of HIV,” World Health Organization. https://www.who.int/hiv/topics/mtct/about/en/
- ↑ 7.0 7.1 7.2 7.3 7.4 Merson, M., O’Malley, J., Serwadda, D., Apsuk, C. The history and challenge of HIV prevention. Lancet. 2008. 372, 475-488.DOI:10.1016/S0140- 6736(08)60884-3
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Roser, M. and Ritchie, H. “HIV/AIDS,” Our World in Data. November, 2019. https://ourworldindata.org/hiv-aids
- ↑ Ouynag, H. and Caron, S. “The City Losing its Children to HIV,” New York Times. March 31, 2021. https://www.nytimes.com/2021/03/31/magazine/pakistan-hiv.html?smid=fb-nytimes&smtyp=cur&fbclid=IwAR2V2M1rF2omhLWvWStR1upUfkfl_81PpM-MLbDolv80ipTDvmwMpp2E6gA
- ↑ 10.0 10.1 10.2 “U.S Statistics” HIV.gov. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
- ↑ 11.0 11.1 11.2 “HIV Treatment – The Basics,” HIV.gov. September 24, 2020. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics
- ↑ Riddell, J. Amico, ., Mayer, K. HIV Preexposure Prophylaxis: A Review. JAMA Netowork 2018. 319(12). https://pubmed.ncbi.nlm.nih.gov/29584848/
- ↑ “Lifetime Risk of HIV Diagnosis,” Centers for Disease Control. February 23, 2016. https://www.cdc.gov/nchhstp/newsroom/2016/croi-press-release-risk.html
- ↑ 14.0 14.1 Linda Vilarosa, “America’s Hidden HIV Epidemic,” New York Times. June 6, 2017. https://www.nytimes.com/2017/06/06/magazine/americas-hidden-hiv-epidemic.html
- ↑ 15.0 15.1 Engelman, A., Cherepanov, P. The structural biology of HIV-1: mechanistic and therapeutic insights. Nature Reviews Microbiology. 2012. 10, 279–290. https://doi.org/10.1038/nrmicro2747
- ↑ 16.0 16.1 16.2 16.3 16.4 Kirchhoff, F. HIV Life Cycle. Encyclopedia of AIDS. DOI 10.1007/978-1-4614-9610-6_60-1
- ↑ 17.0 17.1 Mascola, J. and Montefiori, D. The Role of Antibodies in HIV Vaccines. Annual Review of Immunology. 2010. 28, 413-444. https://doi.org/10.1146/annurev-immunol-030409-101256
- ↑ 18.0 18.1 18.2 18.3 Wibmer CK, Gorman J, Ozorowski G, et al. Structure and Recognition of a Novel HIV-1 gp120-gp41 Interface Antibody that Caused MPER Exposure through Viral Escape. PLoS Pathogens. 2017. 3(1):e1006074.. doi:10.1371/journal.ppat.1006074
- ↑ 19.0 19.1 Pancera M, Majeed S, Ban YE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proceedings National Academy of Science USA. 2010.107(3):1166-71. doi: 10.1073/pnas.0911004107.
- ↑ 20.0 20.1 Anderson, J., Javien, J., Nolta, J., Bauer, G. Preintegration HIV Inhibition by a Combination Lentiviral Vector Containing a Chimeric TRIM5a Protein, a CCR5 shRNA, and a TAR Decoy. American Society of Gene and Cell Therapy. 2009. 17(12). http://europepmc.org/article/PMC/2814390
- ↑ Haynes, B. and Burton, D. Developing an HIV Vaccine. Science. 2017. 355(6330). DOI: 10.1126/science.aan0662
- ↑ Gulick, R. and Flexner, C. Long-Acting HIV Drugs for Treatment and Prevention. 2019. Annual Reviews of Medicine. 70, 137-50. https://doi.org/10.1146/annurev- med- 041217- 013717
- ↑ Cihlar, T. and Fordyce, M. Current Status and Prospects of HIV Treatment. 2016. Current Opinion in Virology. 18, 50-56.
- ↑ 24.0 24.1 Brehm, J., Scott, Y., Koontz, D., Perry, S. et al. Zidovudine (AZT) Monotherapy Selects for the A360V Mutation in the Connection Domain of HIV-1 Reverse Transcriptase. 2012. 7(2). https://doi.org/10.131/journal.pone.0031558
- ↑ N., Funes, H., Blas-Garcia, A., Galindo, M. et al.. Efavirenz and the CNS: what we already know and questions that need to be answered. Journal of Antimicrobial Chemotherapy. 70(10) 2693–2708, https://doi.org/10.1093/jac/dkv183
- ↑ Thenin-Houssier S, de Vera IM, Pedro-Rosa L, Brady A, Richard A, Konnick B, Opp S, Buffone C, Fuhrmann J, Kota S, et al. Ebselen, a Small-Molecule Capsid Inhibitor of HIV-1 Replication. Antimicrob Agents Chemother. 2016. 60(4). doi: 10.1128/AAC.02574-15.
- ↑ 27.0 27.1 Haynes, B. and Burton, D. Developing an HIV Vaccine. Science. 2017. 355(6330). DOI: 10.1126/science.aan0662
- ↑ “HIV Vaccines,” Iavi. https://www.iavi.org/our-science/hiv-vaccines
- ↑ 29.0 29.1 Hsu, D. and O’Connell, R. Progress in HIV Vaccine Development. Taylor and Francis Group. 2017. 13(5). https://doi.org/10.1080/21645515.2016.1276138
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDeveloping_Vaccines - ↑ Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28(2) doi:10.1097/QAD.0000000000000106
- ↑ Huang, D., Tran, J.T., Olson, A. et al. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. Nat Commun 11, 5850 (2020). https://doi.org/10.1038/s41467-020-19650-8
- ↑ HIV Trials Network, “Types of Vaccines,” Fred Hutchinson. https://www.hvtn.org/en/science/hiv-vaccine-basics/types-vaccines.html
- ↑ 34.0 34.1 34.2 Hsu, D. and O’Connell, R. Progress in HIV Vaccine Development. Taylor and Francis Group. 2017. 13(5). https://doi.org/10.1080/21645515.2016.1276138
- ↑ Parks, C., Picker, L., King, C. Development of replication-competent viral vectors for HIV vaccine delivery. Curr Opin HIV AIDS. 2013;8(5) doi:10.1097/COH.0b013e328363d389
- ↑ Baden, L, Stieh, D., Sarnecki, M., et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet 2020. 7(10). https://doi.org/10.1016/S2352-3018(20)30229-0
