Hantaviruses: Old World vs. New World
Page written/researched by Raphael Antonio Melo
Hantaviruses
Background
Hantaviruses belong to the viral family Bunyaviradae, of which there are five genera: orthbunyavirus, phlebovirus, nairovirus, tospovirus, and hantavirus (HTV).[1] There are around 36 known species of HTV that currently exist in the world, with the number only increasing as more are discovered.[2] Hantaviruses are typically able to infect humans via urine and fecal matter of rodents.[3]
Rodents are the main vector of this disease into humans.[4] Transmission from human to human is far more rare than rodent to human, although it is not impossible.[5] There are many other strains of HTV that may infect other species of animals, but in regards to known vectors of medical importance, only rodent species are known to be able to cause infection in humans.[1] Infected aerosolized particles may enter the human body through mucous membranes in the eyes, nose, and mouth, and through breaks in the skin, for example, from a rodent bite.[1] When bedding covered in rodent waste is disturbed, the waste particles become aerosolized and are able to be inhaled, potentially leading to infection.[3]
The first recorded HTV species was documented as the Hantaan virus, an Old-World variant, between 1951 and 1953 in Korea, and was the first species of many.[4] Infecting more than 3000 soldiers during the Korean war, the Hantaan virus outbreak was located near the Hantan river, providing the virus with its name that would later represent all future species falling under the genus hantavirus.[6][7]
In regards to healthcare, there is no effective cure or treatment for HTV.[8] Patients will typically receive a broad range of antibiotic therapies and supportive care while being closely monitored.[9] In regards to treatment based on the type of HTV, there are generally different procedures and drugs administered based on whether or not the species of HTV is “Old World” or “New World.”[9] HTV human infections may result in two possible diseases: hemorrhagic fever with renal syndrome (HFRS) and HPS hantavirus pulmonary syndrome.[10] What determines whether the infection yields HFRS or HPS is the type of capillary bed that is infected.[11] For HFRS, renal medulla capillaries are the origin of infection while for HPS, the pulmonary capillaries are the target.[11] Despite this, most initial symptoms tend to be the same for both syndromes, including typical flu-like symptoms in addition to aggressive fevers, malaise, and myalgia.[11] Additional symptoms include increased vascular permeability which can lead to hypotension, thrombocytopenia, and leukocytosis with a left shift, meaning that an abundance of neutrophils are destroyed at the site of infection, causing an increase in production in the bone marrow.[11][12]
Viral Life Cycle and Structure

HTV virions typically infect endothelial, epithelial, macrophage, follicular dendritic, and lymphocyte cells.[13] Using glycoproteins, the virions attach to the host cell’s surface membrane receptors and enter the cell via endocytosis.[14] The most common receptors that allow for entry of the virion are β1 integrins, with some exceptions involving β3 receptors.[9] Upon cellular uptake, the virions are enclosed in endosomes where the viral capsule is immediately uncoated.[9] The L, M, and S mRNA segments are set up for transcription via the viral RdSp enzyme, and subsequent translation of the L and S mRNA occurs on free floating ribosomes, while the M mRNA undergoes translation on membrane-bound ribosomes.[14] Assembly of all virion components at the golgi apparatus means the virus exclusively replicates within the host’s cytoplasm.[10] Beyond this, little more than speculation exists on how HTV RNPs are synthesized, trafficked to the golgi, and what drives the budding mechanisms of the golgi.[9] Further speculation of alternative assembly methods for new world hantaviruses could point towards the plasma membrane as a potential assembly site.[9] Golgi vesicles containing newly made viral particles then fuse with the host cell’s plasma membrane, and are then released into the extracellular environment.[9]
Genome
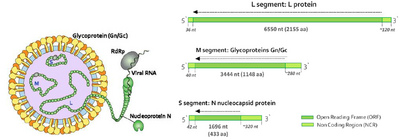
Hantaviruses are negative-sensed, enveloped, single-stranded RNA viruses.[11][13] They have 3 segments of RNA within their genome, Small (S) , Medium (M), and Large (L).[15][13] Each mRNA segment’s open reading frame is placed in between much smaller non-coding regions that are capped.[13] The S RNA segment is 2059 to 2060 nucleotides long, the M segment is 3696 nucleotides, and the L segment is about 6562 nucleotides.[16] The L segment plays a key protein in viral replication and codes for the production of L proteins, which facilitate transcriptase and replicase functions.[14] Additionally, L proteins are thought to have a role in endonuclease cleavage of cellular mRNAs to produce primer caps used for transcription initiation of viral mRNAs in a process known as “cap snatching.”[16] The M segment codes for the glycoprotein precursors Gn and Gc, also known as G1 and G2 proteins respectively.[14] These proteins later form the glycoprotein outer membrane matrix and allow the virus to bind to host cell receptors.[14] The S segment codes for N proteins, which serve a variety of functions needed to complete the viral life cycle.[14] Arguably the most important of the proteins, N proteins aid in translation, trafficking, assembly, the possible modulation of the host’s immune system, and serve as the building material needed to form the viral nucleocapsid.[14]
Old World Hantaviruses
Hantaviruses in Asia and Europe
The Hantaan, Dobrava, Saaremaa, Seoul, and Puumala strains may lead to infections causing HFRS, and are found throughout parts of Asia, Africa, Russia, and Europe, with each strain retaining a locational and original name.[17] It is debated when the first actual cases of HFRS were first documented, when eastern siberian medical examination records detailed a viral disease in humans that is what we now know as HFRS.[7] Even greater was the discovery of a Chinese clinical record reporting a similar ailment dated even further back, around 960 AD.[7] HFRS has a significant historical correlation with armed conflict due to poor hygiene and lack of resources during wartime such as records pointing to a syndrome of 20th century wars and conflicts known as “field nephritis” or “trench nephritis.”[18] Field nephritis shares similar characteristics with what we now know to be HFRS, and is theorized to possibly refer to some past cases of HFRS prior to its recognition as a disease in the 1930s-1940s.[18] This syndrome was experienced by the German and allied trenches of WWI during the battle of Flanders, Japanese troops in the 1930’s during the Manchurian operation in China, and again in Europe during WWII when Finnish troops engaged German troops in Lapland in 1942.[7] That being said, most of these records also contain certain additional symptoms or factors that directly contradict known symptoms of HFRS, such as renal swelling, lack of renal hemorrhaging, and glomerular damage in the kidneys.[19] The exception to this being the outbreak in Lapland, Finland, where it was found that voles served as the vector for what later became known as the Puumala virus, a less lethal yet extremely prominent species of HTV.[20]
Reports of Soldiers engaging in training exercises across the globe from the 1990s to the mid 2000s has also turned up outbreaks of HTV.[21] Most notable, 14 U.S. Marines contracted the disease while training in South Korea in 1986, and then 4 more again at the Korean DMZ in 2005.[20] Additionally, some 700 Swedish soldiers fell ill to the Puumala virus and a study conducted found that military populations were at a greater risk than civilian populations of becoming infected specifically with the Puumala virus.[20] Relevancy to Old World HTV infections hasn’t stopped with the turn of the century either, with some suspecting it may resurface during the 2022 ongoing installment of the Russo-Ukrainian war, evidenced by 1.6% of Ukraine’s healthy population having HTV antibodies.[22]
As mentioned earlier, the first official documentation and discovery of the first HTV outbreak occurred in Korea in 1951 when roughly 3000 UN and Korean troops became infected with the Hantaan strain, named for the nearby Hantan river.[7] It was generally assumed that only farmers in Eurasia, soldiers, and people who resided in rural areas were the susceptible demographic, however with further surveillance it was demonstrated that both rural and urban areas on a global scale could serve as grounds for infection.[7]
In particular, the most prominent strains associated with the Old World title are the Amur/Soochong virus from the Russian Far East, the Dobrava-Belgrade virus with Balkan origins, the Puumala virus found in Europe and Asia but named after a finnish municipality, the Luxi virus from China, the Saaremaa virus from Europe, and the Seoul virus of South Korea.[20] The most prevalent current HTV virus under the Old World classification is the Puumala virus according to a 2023 report on hantavirus cases in 2020: it saw 98% of all HTV infections across 28 countries in Europe.[23] Other prevalent Old World viruses include the Seoul virus, the Hantaan virus, and Dobrava-Belgrade virus.[17]
Hemorrhagic Fever with Renal Syndrome
HFRS is difficult to diagnose the disease from a clinical standpoint, and will often require serology tests to fully conclude a diagnosis.[24] A substantial increase in IgG antibodies and the presence of IgM antibodies within the body provide solid evidence for an acute HTV infection.[24]
HFRS are technically a group of very similar diseases that all hail from a small subset of hantaviruses: Korean hemorrhagic fever, epidemic hemorrhagic fever, and nephropathia epidemica.[25] The species of Hantavirus that result in these syndromes include Hantaan, Dobrava, Saaremaa, Seoul, and the Puumala strain.[25] Different Old World strains are known to cause HFRS with varying degrees of lethality depending on which type of strain is responsible for the infection.[26]
Typically, HFRS is divided into 5 distinct phases that the patient may experience.[11] The first phase involves an incubation period of 2 to 4 weeks, followed by a second, febrile phase that involves headache, backache, high fever, abdominal pains, chills, nausea and vomiting.[27] Somnolence, lethargy, and visual distortion and blurring have also often been reported in addition, and the end of this phase sees conjunctival hemorrhages in the eyes.[11] The second stage can last as little as a couple of hours to a day or two, and involves hypotension.[11] In some cases shock is also present, and about ⅓ of all deaths of HFRS result from imminent shock.[11] Further hemorrhaging, thrombocytopenia and leukocytosis are characteristic of this phase especially.[28]
The third phase, the oliguric phase, lasts about 3 to 7 days, and generally sees a decline in kidney function yielding abdominal and back pains.[28]
This phase results in half of all HFRS associated deaths, and tends to be the most lethal of the five phases with renal hemorrhagic and dysfunction occurring.[28]
The fourth phase, the polyuric phase, sees a reversal of the third phase, with a return of kidney function. This leads to the diuretic phase which is typically a sign of recovery, and can last anywhere from days to weeks, and sees a massive increase in micturition in the patient.[28] Recovery can take up to half a year to complete, and there are very rarely lingering symptoms or complications once recovery is complete.[28]
New World Hantaviruses
Hantaviruses in the Americas

Prior to 1993, the classification of New World and Old World Hantaviruses did not exist.[29] This changed when in May of 1993, the first ever outbreak of a new series of hantavirus species occurred in the United States.[29] In an attempt to quickly locate disease vectors and pin down the identity of this new disease as the outbreak was occuring, medical professionals and researchers discovered that some lung tissues from bodies killed or afflicted by previously unknown lung diseases matched the pathogenesis and phenotypes present in the lungs of the patients involved with the 1993 outbreak.[30] The first outbreak involved the first New World species which was originally dubbed the Four Corners virus but later changed to the Sin Nombre virus after it no locational name was agreed upon due to fear of lack of tourism and negative public image.[30] Since then, new HTV outbreaks discovered in the Americas have been dubbed as New World hantaviruses and an associated disease known as hantavirus pulmonary syndrome (HPS) is known to occur as a result of infection.[29]
Current relevant research and outbreaks of New World hantaviruses in the last couple years informs us that New World viruses are far more prevalent than Old World viruses in the Americas.[31] The most common species are the Sin Nombre virus and the Andes virus, which In Los Santos, Panama, July of 2022, 17 total cases were reported, 12 of which later developed the disease HPS as a result of infection.[14][32]
Hantavirus Pulmonary Syndrome
New World HTV infections tend to cause a syndrome known as Hantavirus Pulmonary Syndrome (HPS), or Hantavirus Cardiopulmonary Syndrome (HCPS).[33] HPS occurs when an HTV virus infects the lungs, the pulmonary capillaries and blood vessels of the lungs become leaky, which can lead to the lungs filling up with fluid, causing breathing difficulties.[33] When an HTV virus infects the heart, the damage caused by the infection can lead to low blood pressure, causing shock.[33] Almost all deaths related to HPS are a result of shock rather than respiratory failure.[34] Lack of oxygen being sent to tissues and organs as a bi-product of low blood pressure can quickly lead to death when left untreated.[35] HPS tends to be far more lethal than HFRS with a mortality rate existing around 30 to 50%.[36]
Similar to HFRS, HPS has multiple phases of disease, but instead of 5 there are 3: prodromal, cardiopulmonary and convalescent.[37] The prodromal phase involves a very similar set of symptoms to the first phase of HFRS, with symptoms including high fever, chills, myalgia, nausea, headache,vomiting, abdominal pain and diarrhea.[37] The second phase, the cardiopulmonary phase, coughing may develop and get worse over time, rapid heart rate, and shortness of breath.[38] Further into this phase, patients may develop acute non-cardiac pulmonary edema, lactic-acidosis, and experience hypotension.[14] These conditions also may worsen to the point that respiratory failure occurs, and mechanical ventilation may become necessary.[14] The final stage involves slow convalescence, with patients typically experiencing fatigue and weakness.[14]
Further Treatments and Prevention
Critical care management of HTV infections that yield both HFRS and HPS/HCPS stress the avoidance of fluid overload due to the possibility of increasing fluid buildup in the lungs or swelling of the kidneys.[39][40] Pressors to maintain cardiac output are also essential during the treatment process, and in the most extreme cases, the use of extracorporeal membrane oxygenation.[40]
There is no current vaccine or cure for HTV infections.[39] One of the reasons why a vaccine is not currently being pursued is due to the dangers associated with mass-production of a virus under strict containment.[41] Some work has been previously done but a major roadblock is that the smallpox vaccine greatly reduced the efficacy of the prototype HTV vaccine.[41] Many attempts to create effective vaccines have approached using plasmids and viral genomes in particular to stimulate the desired type and number of antibodies, however, usually to no avail.[42][40] Given the number of HTV infections that occur each year, it is necessary for further research to fully discern a proper and decisive method of treatment, however that number of infections is not large enough to warrant further research towards the particular production of a vaccine.
The antiviral drug ribavirin, also known as virazole, is a drug used for HTV infections, and efficacy of the drug depends entirely on how close to the initial time of infection the drug is taken as it targets the replication cycle.[43][44] The general efficacy of ribavirin is heavily debated based on a slew of clinical trials from the 90s and early 2000s that yielded relatively non-conclusive results in regards to effectiveness.[45] Despite that, it is one of the most broad-range antiviral agents available currently and is an effective early stage treatment for HPS, especially when combined with other supportive drugs.[46][47] Ribavirin is able to successfully prevent growth in mammalian cells, and has other cytostatic properties that allow it to cause hyper mutations in RNA viruses, and disrupt the lifecycle of DNA viruses at the replication stage.[46]
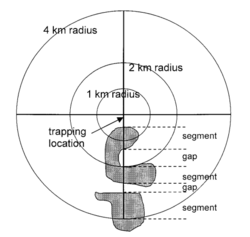
In regards to preventing outbreaks from an ecological standpoint, some research into organismal ecology that was being carried out around the same time of the initial outbreak in 1993 was consistent with later research in 2001, which concluded that the composition of the landscape had a positive correlation on the presence of Sin Nombre virus in the deer mice vector.[48] The 2001 study ultimately concluded that the spatial structure of landscape data is rather important for epidemiology and the associated models predicting emerging infectious diseases and disease transmission.[49] Mice were trapped and tested for seroprevalence in an 8 kilometer radius in Canada at 101 different sites across the country.[49] Landscape conditions, such as configuration and composition of the landscape itself, that promote significant presence of Sin Nombre in deer mice in the later 2001 study matched the conditions of the American Southwest in the months leading up to the Four Corners outbreak in May of 1993.[49][48]
Beyond macro ecological prevention and vaccinations, more localized precautions can be taken.[50] Local prevention of hantavirus involves preventing the disease vector itself from getting in contact with the individual, such as taking measures to seal up homes, maintain clean living environments, and providing traps for rodents.[50]
References
- ↑ 1.0 1.1 1.2 Centers for Disease Control and Prevention. (2021, November 16). CDC - Hantavirus. Centers for Disease Control and Prevention. https://www.cdc.gov/hantavirus/index.html
- ↑ Hantavirus (2021) National Collaborating Centre for Infectious Diseases. Available at: https://nccid.ca/debrief/hantavirus/ (Accessed: 14 April 2024).https://nccid.ca/debrief/hantavirus/
- ↑ 3.0 3.1 Clement, J., Maes, P. and Van Ranst, M., 2006. Hantaviruses in the old and new world. Perspectives in Medical Virology, 16, pp.161-177. https://www.sciencedirect.com/science/article/abs/pii/S0168706906160085
- ↑ 4.0 4.1 Ho Wang Lee, Pyund Woo Lee, Karl M. Johnson, Isolation of the Etiologic Agent of Korean Hemorrhagic Fever, The Journal of Infectious Diseases, Volume 137, Issue 3, March 1978, Pages 298–308, https://doi.org/10.1093/infdis/137.3.298
- ↑ Wells, R.M., Estani, S.S., Yadon, Z.E., Enria, D., Padula, P., Pini, N., Mills, J.N., Peters, C.J. and Segura, E.L., 1997. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerging infectious diseases, 3(2), p.171.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2627608/
- ↑ Mir, M., 2010. Hantaviruses. Clinics in laboratory medicine, 30(1), p.67.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2880890/
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Johnson, K.M., 2001. Hantaviruses: history and overview. Hantaviruses, pp.1-14.inger.com/chapter/10.1007/978-3-642-56753-7_1
- ↑ Diagnosis & treatment (2012) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hantavirus/hps/diagnosis.html#:~:text=There%20is%20no%20specific%20treatment,unit%2C%20they%20may%20do%20better.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Jonsson, C.B., Hooper, J. and Mertz, G., 2008. Treatment of hantavirus pulmonary syndrome. Antiviral research, 78(1), pp.162-169.https://www.sciencedirect.com/science/article/pii/S016635420700438X.
- ↑ 10.0 10.1 Parvate, A., Williams, E. P., Taylor, M. K., Chu, Y. K., Lanman, J., Saphire, E. O., & Jonsson, C. B. (2019). Diverse Morphology and Structural Features of Old and New World Hantaviruses. Viruses, 11(9), 862.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6783877/#:~:text=Hantaviruses%20endemic%20to%20Eurasia%20are,hantavirus%20pulmonary%20syndrome%20(HPS)
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 Avšič-Županc, T., Saksida, A. and Korva, M., 2019. Hantavirus infections. Clinical Microbiology and Infection, 21, pp.e6-e16.https://www.sciencedirect.com/science/article/pii/S016635420700438X
- ↑ Ishimine, N., Honda, T., Yoshizawa, A., Kawasaki, K., Sugano, M., Kobayashi, Y., & Matsumoto, T. (2013). Combination of white blood cell count and left shift level real-timely reflects a course of bacterial infection. Journal of clinical laboratory analysis, 27(5), 407–411. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6807635/#:~:text=Therefore%2C%20left%20shift%20indicates%20that,neutrophils%20to%20the%20infected%20site
- ↑ 13.0 13.1 13.2 13.3 Razzauti Sanfeliu, M., 2012. Microevolution of Puumala hantavirus in its host, the bank vole (Myodes glareolus).https://www.researchgate.net/publication/282867889_Microevolution_of_Puumala_hantavirus_in_its_host_the_bank_vole_Myodes_glareolus
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 Jonsson, C.B., Figueiredo, L.T.M. and Vapalahti, O., 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clinical microbiology reviews, 23(2), pp.412-441.https://journals.asm.org/doi/full/10.1128/cmr.00062-09
- ↑ Schmaljohn, C.S. and Dalrymple, J.M., 1983. Analysis of Hantaan virus RNA: evidence for a new genus of Bunyaviridae. Virology, 131(2), pp.482-491..https://www.sciencedirect.com/science/article/pii/0042682283905147
- ↑ 16.0 16.1 Virology (2012) Centers for Disease Control and Prevention. Available at:https://www.cdc.gov/hantavirus/technical/hanta/virology.html#:~:text=The%20L%20RNA%20is%206562,2059%20to%202060%20nt%20long.
- ↑ 17.0 17.1 JWei, X., Li, X., Song, S., Wen, X., Jin, T., Zhao, C., Wu, X., Liu, K. and Shao, Z., 2022. Trends and focuses of hantavirus researches: a global bibliometric analysis and visualization from 1980 to 2020. Archives of Public Health, 80(1), p.218.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9526533/#:~:text=The%20Sin%20Nombre%20virus%20(SNV)%20and%20Andes%20virus%20
- ↑ 18.0 18.1 Atenstaedt RL: The response to the trench diseases in World War I: a triumph of public health science. Public Health 2007; https://www.sciencedirect.com/science/article/pii/S0033350607000352?via%3Dihub
- ↑ Atenstaedt RL: The medical response to trench nephritis in World War one. Kidney Int 2006; 70(4): 635–40.doi: 10.1038/sj.ki.5001618.
- ↑ 20.0 20.1 20.2 20.3 Jukka Mustonen, Heikki Henttonen, Antti Vaheri, Hantavirus Infections among Military Forces, Military Medicine, Volume 189, Issue 3-4, March/April 2024 https://academic.oup.com/milmed/article/189/3-4/551/7222231
- ↑ Markotic A, LeDuc JW, Hlaca D, et al. : Hantaviruses are likely threat to NATO forces in Bosnia and Herzegovina and Croatia. Nat Med 1996; 2(3): 269–70.doi: 10.1038/nm0396-269.
- ↑ Lozynskyi I, Shulgan A, Zarichna O, et al. : Seroprevalence of old hantaviruses and Crimean Congo hemorrhagic fever viruses in human populations in Nortwestern Ukraine. Front Cell Infect Microbiol 2020; 10: 589464.doi: 10.3389/fcimb.2020.589464.
- ↑ Hantavirus infection - annual epidemiological report for 2020 (2023) European Centre for Disease Prevention and Control. Available at: https://www.ecdc.europa.eu/en/publications-data/hantavirus-infection-annual-epidemiological-report-2020#:~:text=For%202020%2C%2028%20countries%20reported,no%20obvious%20long%2Dterm%20trend
- ↑ 24.0 24.1 Lee HW, van der Groen G. Hemorrhagic fever with renal syndrome. Progress in Medical virology. Fortschritte der Medizinischen Virusforschung. Progres en Virologie Medicale. https://europepmc.org/article/med/2573914
- ↑ 25.0 25.1 CDC - hemorrhagic fever with renal syndrome (HFRS) - hantavirus (2017) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hantavirus/hfrs/index.html#:~:text=Suggested%20Reading-,What%20is%20hemorrhagic%20fever%20with%20renal%20syndrome%3F,hemorrhagic%20fever%2C%20and%20nephropathia%20epidemica
- ↑ Bi, Z., Formenty, P.B. and Roth, C.E., 2008. Hantavirus infection: a review and global update. The Journal of Infection in Developing Countries, 2(01), pp.003-023.https://jidc.org/index.php/journal/article/view/19736383
- ↑ Linderholm, M. and Elgh, F., 2001. Clinical characteristics of hantavirus infections on the Eurasian continent. Hantaviruses, pp.135-151.https://link.springer.com/chapter/10.1007/978-3-642-56753-7_8
- ↑ 28.0 28.1 28.2 28.3 28.4 Krüger, D.H., Ulrich, R. and Lundkvist, Å., 2001. Hantavirus infections and their prevention. Microbes and infection, 3(13), pp.1129-1144.https://www.sciencedirect.com/science/article/pii/S1286457901014745
- ↑ 29.0 29.1 29.2 Sah, R., Mohanty, A., Rohilla, R., Padhi, B. K., Chandran, D., Chakraborty, C., & Dhama, K. (2022). Recent outbreaks of hantavirus-a very lethal and zoonotic virus - An update and counteracting strategies. International journal of surgery open, 49, 100582.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9686047/
- ↑ 30.0 30.1 CDC - history of hantavirus pulmonary syndrome (HPS) - hantavirus (2020) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hantavirus/outbreaks/history.html
- ↑ Mertz, G.J., Hjelle, B., Crowley, M., Iwamoto, G., Tomicic, V. and Vial, P.A., 2006. Diagnosis and treatment of new world hantavirus infections. Current opinion in infectious diseases, 19(5), pp.437-442.https://journals.lww.com/co-infectiousdiseases/abstract/2006/10000/diagnosis_and_treatment_of_new_world_hantavirus.7.aspx
- ↑ Sah, R., Mohanty, A., Rohilla, R., Padhi, B. K., Chandran, D., Chakraborty, C., & Dhama, K. (2022). Recent outbreaks of hantavirus-a very lethal and zoonotic virus - An update and counteracting strategies. International journal of surgery open, 49, 100582.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9686047/#:~:text=Andes%20virus%20has%20been%20proved,Santos%2C%20Panama%20%5B10%5D.
- ↑ 33.0 33.1 33.2 Association, A.L. (no date) Learn about hantavirus pulmonary syndrome, American Lung Association. Available at: https://www.lung.org/lung-health-diseases/lung-disease-lookup/hantavirus-pulmonary-syndrome/learn-about-hantavirus-pulmonary-syndrome-hps#:~:text=Hantaviruses%20infect%20people%20when%20they,making%20it%20difficult%20to%20breathe
- ↑ Saggioro, F.P., Rossi, M.A., Duarte, M.I.S., Martin, C.C.S., Alves, V.A., Moreli, M.L., Figueiredo, L.T.M., Moreira, J.E., Borges, A.A. and Neder, L., 2007. Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantavirus pulmonary syndrome. The Journal of infectious diseases, 195(10), pp.1541-1549.https://academic.oup.com/jid/article/195/10/1541/2192094
- ↑ Hallin, G.W., Simpson, S.Q., Crowell, R.E., James, D.S., Koster, F.T., Mertz, G.J. and Levy, H., 1996. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Critical care medicine, 24(2), pp.252-258.https://journals.lww.com/ccmjournal/abstract/1996/02000/cardiopulmonary_manifestations_of_hantavirus.12.aspx
- ↑ Sah, R., Mohanty, A., Rohilla, R., Padhi, B. K., Chandran, D., Chakraborty, C., & Dhama, K. (2022). Recent outbreaks of hantavirus-a very lethal and zoonotic virus - An update and counteracting strategies. International journal of surgery open, 49, 100582.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9686047/
- ↑ 37.0 37.1 Enria, D.A., Briggiler, A.M., Pini, N. and Levis, S., 2001. Clinical manifestations of New World hantaviruses. Hantaviruses, pp.117-134.https://link.springer.com/chapter/10.1007/978-3-642-56753-7_7
- ↑ Ketai, L.H., Williamson, M.R., Telepak, R.J., Levy, H., Koster, F.T., Nolte, K.B. and Allen, S.E., 1994. Hantavirus pulmonary syndrome: radiographic findings in 16 patients. Radiology, 191(3), pp.665-668.https://pubs.rsna.org/doi/abs/10.1148/radiology.191.3.8184043
- ↑ 39.0 39.1 CDC - technical/clinical information: HPS treatment - hantavirus (2012) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hantavirus/technical/hps/treatment.html#:~:text=ICU%20management%20should%20include%20careful,the%20potential%20for%20capillary%20leakage
- ↑ 40.0 40.1 40.2 Dheerasekara, K., Sumathipala, S. & Muthugala, R. Hantavirus Infections—Treatment and Prevention. Curr Treat Options Infect Dis 12, 410–421 (2020). https://doi.org/10.1007/s40506-020-00236-3
- ↑ 41.0 41.1 Hammerbeck, C.D. and Hooper, J.W., 2016. Hantavirus vaccines. In New Generation Vaccines (pp. 933-941). CRC Press.https://www.taylorfrancis.com/chapters/edit/10.3109/9781420060744-87/hantavirus-vaccines-christopher-hammerbeck-jay-hooper
- ↑ Song, J. Y., Woo, H. J., Cheong, H. J., Noh, J. Y., Baek, L. J., & Kim, W. J. (2016). Long-term immunogenicity and safety of inactivated Hantaan virus vaccine (Hantavax™) in healthy adults. Vaccine, 34(10), 1289–1295. https://doi.org/10.1016/j.vaccine.2016.01.031
- ↑ Huggins, J.W.; Kim, G.R.; Brand, O.M.; McKee, K.T., Jr. Ribavirin therapy for hantaan virus infection in suckling mice. J. Infect. Dis. 1986, 153, 489–497. [Google Scholar]
- ↑ Brocato RL, Hooper JW. Progress on the Prevention and Treatment of Hantavirus Disease. Viruses. 2019; 11(7):610. https://doi.org/10.3390/v11070610
- ↑ Mertz, G.J.; Miedzinski, L.; Goade, D.; Pavia, A.T.; Hjelle, B.; Hansbarger, C.O.; Levy, H.; Koster, F.T.; Baum, K.; Lindemulder, A.; et al. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in north america. Clin. Infect. Dis. 2004, 39, 1307–1313. [Google Scholar]
- ↑ 46.0 46.1 Enna, S.J. and Bylund, D.B. (2008) Xpharm: The Comprehensive Pharmacology Reference: Reference Work. Issy-les-Moulineaux: Elsevier Masson.https://www.sciencedirect.com/topics/chemistry/ribavirin#:~:text=The%20antiviral%20drug%20Ribavirin%20
- ↑ Ribavirin: Medlineplus drug information (no date) MedlinePlus. Available at: https://medlineplus.gov/druginfo/meds/a605018.html
- ↑ 48.0 48.1 Carver, S., Mills, J. N., Parmenter, C. A., Parmenter, R. R., Richardson, K. S., Harris, R. L., Douglass, R. J., Kuenzi, A. J., & Luis, A. D. (2015). Toward a Mechanistic Understanding of Environmentally Forced Zoonotic Disease Emergence: Sin Nombre Hantavirus. Bioscience, 65(7), 651–666. https://doi.org/10.1093/biosci/biv047
- ↑ 49.0 49.1 49.2 Langlois, J.P., Fahrig, L., Merriam, G. et al. Landscape structure influences continental distribution of hantavirus in deer mice. Landscape Ecology 16, 255–266 (2001). https://doi.org/10.1023/A:1011148316537
- ↑ 50.0 50.1 Prevention (2012) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hantavirus/hps/prevention.html
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
