Heavy Metal Toxicity and Resistance: Difference between revisions
No edit summary |
No edit summary |
||
| Line 30: | Line 30: | ||
Metal toxicity differs from [http://en.wikipedia.org/wiki/Antibiotic antibiotics] mostly because there are no components to modify [[#References|(1)]], but there are still a couple of ways the cell can defend itself. | Metal toxicity differs from [http://en.wikipedia.org/wiki/Antibiotic antibiotics] mostly because there are no components to modify [[#References|(1)]], but there are still a couple of ways the cell can defend itself. | ||
==Efflux or sequestration== | |||
Cations can be pumped out of the cell with low ATP expense, or sequestered by chelates like thiol-containing compounds at approximately 16 ATP, an option that is only cost-effective at low metal concentrations [[#References|(3)]]. | |||
==Reduction to less toxic state== | |||
Some cations can be reduced to a less detrimental state if its reduction potential is within physiological conditions (-421 to 808mV), but sometimes the reduced state may be more toxic or insoluble [[#References|(1)]]. Extracellular electron transfer has also been hypothetically linked to [http://microbewiki.kenyon.edu/index.php/The_role_of_Geobacter_spp._in_Bioremediation_and_Microbial_Fuel_Cells nanowires], and is an area of active research [[#References|(3)]]. | |||
==Biofilm formation== | |||
Some bacteria like ''P. aeruginosa'' can form [http://en.wikipedia.org/wiki/Biofilm biofilms], where there is a heterogeneous population of cells with altered physiology [[#References|(3)]]. For example, those that are nearest to the substrate are usually anoxic and quiescent, and thus experience less ROS production, allowing tolerance to metal [[#References|(19)]]. Even dead cells can sequester metals from live cells of the biofilm [[#References|(20)]]. In a method similar to cell differentiation in eukaryotes, the biofilm can use [http://en.wikipedia.org/wiki/Quorum_sensing quorum sensing] to translate detoxifying enzymes or secrete an [http://en.wikipedia.org/wiki/Extracellular_polymeric_substance extracellular polymeric substance] which biosorbs cations and impedes diffusion [[#References|(21)]]. The exact processes are still being examined, but with many phenotypic variants within a biofilm, the versatility and differentiation may give these bacteria an advantage against heavy metals. | |||
=Closing Word= | |||
Each metal ion has a different method of inflicting damage, while each tolerance mechanism has intrinsic advantages against particular cations. More research will help elucidate this arms race and help scientists harness them for use. Currently, heavy metals do seem like a plausible substitute for antibiotics, but its exact administration and mechanisms must be uncovered before clinical use. | |||
=Morphology= | =Morphology= | ||
Revision as of 23:59, 26 November 2013
Heavy Metal Toxicity and Resistance
Introduction
The widespread use of metals has prompted many microbiologists to examine the relationship between heavy metal toxicity and bacterial resistance. Heavy metals are defined as those which have a density of over 5g/cm3 (1), and thus include many of the transition and coinage metals. The reversion to heavy metal biocide use has been suggested as a possible solution to antibiotic resistance (2). On the other hand, bacteria that are resistant to certain heavy metals have been proposed to be economically useful in biomining and bioremediation (1). The constant race between heavy metal toxicity and bacterial resistance will likely give rise to many ingenious applications in the future, so it is important to understand the underlying mechanisms.
Heavy metal toxicity mechanisms
Heavy metals have been historically used as antimicrobial agents prior to the discovery of antibiotics, and have now been incorporated into coatings, surfaces, and internally placed devices (3). Recent evidence has shown that some metals can work synergistically with antibiotics (Cu with polycide) (4), are effective against multi-drug resistant bacteria, and can disrupt biofilms (5,6). There is a lot of diversity in both metals and the bacteria they affect, but in general toxicity can be broken down into five categories (FIG 1).
Oxidative stress
Within normal bacterial cells, iron is essential as a cofactor in enzymes, but it also available in cells to undergo the Fenton reaction to produce reactive oxygen species (ROS) that are damaging to all biological macromolecules (FIG 2) (7). In normal cells, only 20 μM of Fe2+ is available for Fenton reaction, because the rest are all engaged in coordination interactions with ligands (8). When extracellular Fe, Cu, or Ni is introduced into the cell, an increase in ROS and radicals is observed (2). Thiols within the cell also have a high affinity for reducing metals covalently, and can give rise to ROS (2). In order to buffer the redox state within the cell, antioxidants are depleted, and the cell is more vulnerable to ROS from normal metabolism byproducts. Therefore, the metal’s solubility and the reduction potential correlate with the toxicity within the cell (FIG 3) (9).
Protein activity interference
It has been noted that only a few amino acid alterations near the metal-binding region is required to disable function of an enzyme (10). The metal ions often oxidize residues and lead to a formation of carbonyl groups, and proteins with these marker groups are designated for intracellular degradation (11). In bacteria, a family of Fe-S dehydratases is extremely vulnerable to such ROS-mediated oxidation (12). Furthermore, thiol groups and disulfide groups that are important in substrate binding may also be oxidized by cations (3). There has also been evidence of metal cations displacing wild-type cofactors in enzymes using ionic or molecular mimicry - where the metal species is structurally similar but physiologically disruptive (2). An example of this would be gallium substitution of iron (FIG 4) in ribonucleotide reductase, the first enzyme in DNA replication. Ga has similar ionic radius to Fe, but no other oxidation state that is essential to enzyme activity. This substitution also inhibits transcription of pvds (Fe-responsive transcription regulator) in Pseudomonas aeruginosa, initiating a positive feedback cycle that is toxic to multidrug-resistant organisms and quiescent cells in the center of biofilms (5). This displacement is how Fenton-inactive metals like Ag can release coordinated, intracellular Fe into the cytoplasm and contribute to ROS formation (13).
Nutrient assimilation interference
Metal ions must first enter the cell in order to have an effect (1). They can be transported by fast, unspecific, constitutively-expressed transporters like MIT transporters, or specific, inducible ABC transporters and P-type transporters (1). Some cations can competitively inhibit the assimilation of essential ions by binding to these transporters (14). For example, Cr (IV) reduces sulphate uptake since chromate exhibits molecular mimicry, causing a decrease in intracellular S, and a deficiency in this essential bioelement (15).
Membrane impairment
It has been hypothesized copper and cadmium can cause lipid peroxidation (FIG 4) by increasing mutations that increase the number of unsaturated bonds in fatty acids (16), affecting the integrity of the cytoplasmic membrane. Another method of metal toxicity is through dissipation of the chemiosmotic force or siphoning electrons from the ETC. Silver in Vibrio cholerae have been found to introduce “leakage” of protons across the membrane (17), while other metals are reduced by the quinone molecule and steal electrons away from respiration (3).
Genotoxicity
ROS that was previously created by the Fenton reaction can disrupt DNA replication and lead to cell death (8). While Cr(IV) is a very potent mutagen, lab situations have suggested many additional cations that cause DNA damage (2). Furthermore, copper has been correlated to the destruction of extracellular DNA following cell lysis, and this may inhibit postmortem horizontal gene transfer of resistance via transformation (18).
Resistance Mechanisms
Metal toxicity differs from antibiotics mostly because there are no components to modify (1), but there are still a couple of ways the cell can defend itself.
Efflux or sequestration
Cations can be pumped out of the cell with low ATP expense, or sequestered by chelates like thiol-containing compounds at approximately 16 ATP, an option that is only cost-effective at low metal concentrations (3).
Reduction to less toxic state
Some cations can be reduced to a less detrimental state if its reduction potential is within physiological conditions (-421 to 808mV), but sometimes the reduced state may be more toxic or insoluble (1). Extracellular electron transfer has also been hypothetically linked to nanowires, and is an area of active research (3).
Biofilm formation
Some bacteria like P. aeruginosa can form biofilms, where there is a heterogeneous population of cells with altered physiology (3). For example, those that are nearest to the substrate are usually anoxic and quiescent, and thus experience less ROS production, allowing tolerance to metal (19). Even dead cells can sequester metals from live cells of the biofilm (20). In a method similar to cell differentiation in eukaryotes, the biofilm can use quorum sensing to translate detoxifying enzymes or secrete an extracellular polymeric substance which biosorbs cations and impedes diffusion (21). The exact processes are still being examined, but with many phenotypic variants within a biofilm, the versatility and differentiation may give these bacteria an advantage against heavy metals.
Closing Word
Each metal ion has a different method of inflicting damage, while each tolerance mechanism has intrinsic advantages against particular cations. More research will help elucidate this arms race and help scientists harness them for use. Currently, heavy metals do seem like a plausible substitute for antibiotics, but its exact administration and mechanisms must be uncovered before clinical use.
Morphology
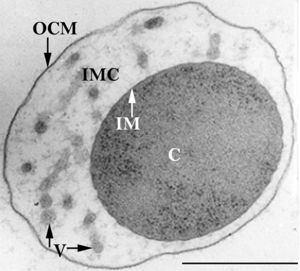
The members of the Ignicoccus genus are motile irregular coccoid cells that range in diameter from 1 to 3 µm. The motility observed is due to the presence of flagella, but unfortunately the polarity of the flagella is not yet fully elucidated. They are known to have an outer-membrane but no S-layer. This is a novel characteristic for these Archaea becauseIgnicoccus are the only known Archaea that have been shown to possess an outer-membrane[2] [10] .
Outer-Membrane
The outer-membrane of Ignicoccus species was found to be composed of various derivatives of the typical lipid archaeol, including the derivative known as caldarchaeol [5] . The outer-membrane is dominated by a pore composed of the Imp1227 protein (Ignicoccus outer membrane protein 1227). The Imp1227 protein forms a large nonamer ring with a predicted pore size of 2nm[7] .
Metabolism
Ignicoccus species are chemolithoautotrophs that use molecular hydrogen as the inorganic electron donor and elemental sulphur as the inorganic terminal electron acceptor[1] . The reduction of the elemental sulphur results in the production of hydrogen sulphide gas.
Ignicoccus are autotrophs in that they fix their own carbon dioxide into organic molecules. The carbon dioxide fixation process they use is a novel process called a dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle that involves 14 different enzymes[8] .
Members of the Ignicoccus genus are able to use ammonium as a nitrogen source.
Growth Conditions
Because members of the Ignicoccus genus are hyperthermophiles and obligate anaerobes, it is not surprising that their growth conditions are very complex. They are grown in a liquid medium known as ½ SME Ignicoccus which is a solution of synthetic sea water which is then made anaerobic.
Grown in this media at their optimal growth temperature of 90C, the members of the Ignicoccus genus typically reach a cell density of ~4x107cells/mL[1] .
The addition of yeast extract to the ½ SME media has been shown to stimulate the growth and increase maximum cell density achieved. The mechanism by which this is achieved is not known[1] .
Symbiosis
Ignicoccus hospitalis is the only member of the genus Ignicoccus that has been shown to have an extensive symbiotic relationship with another organism.
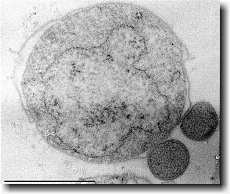
Ignicoccus hospitalis has been shown to engage in symbiosis with Nanoarchaeum equitans . Nanoarchaeum equitans is a very small coccoid species with a cell diameter of 0.4 µm[9] . Genome analysis has provided much of the known information about this species.
To further complicate the symbiotic relationship between both species, it’s been observed that the presence of Nanoarchaeum equitans on the surface of Ignicoccus hospitalis somehow inhibits the cell replication of Ignicoccus hospitalis . How or why this occurs has not yet been elucidated[3] .
Nanoarchaeum equitans
Nanoarchaeum equitans has the smallest non-viral genome ever sequenced at 491kb[9] . Analysis of the genome sequence indicates that 95% of the predicted proteins and stable RNA molecules are somehow involved in repair and replication of the cell and its genome[3] .
Analysis of the genome also showed that Nanoarchaeum equitans lacks nearly all genes known to be required in amino acid, nucleotide, cofactor and lipid metabolism. This is partially supported by the evidence that Nanoarchaeum equitans has been shown to derive its cell membrane from its host Ignicoccus hospitalis cell membrane. The direct contact observed between Nanoarchaeum equitans and Ignicoccus hospitalis is hypothesized to form a pore between the two organisms in order to exchange metabolites or substrates (likely from Ignicoccus hospitalis towards Nanoarchaeum equitans due to the parasitic relationship). The exchange of periplasmic vesicles is not thought to be involved in metabolite or substrate exchange despite the presence of vesicles in the periplasm of Ignicoccus hospitalis .
These analyses of the Nanoarchaeum equitans genome support the fact of the extensive symbiotic relationship between Nanoarchaeum equitans and Ignicoccus hospitalis. However, it has not yet been proven that it is a strictly parasitic relationship and further research may prove that there is a commensal relationship between the two species.
References
(1) Burggraf S., Huber H., Mayer T., Rachel R., Stetter K.O. and Wyschkony I. ” Ignicoccus gen. nov., a novel genus of hyperthermophilic, chemolithoautotrophic Archaea, represented by two new species, Ignicoccus islandicus sp. nov. and Ignicoccus pacificus sp. nov.” International Journal of Systematic and Evolutionary Microbiology, 2000, Volume 50.
(2) Naether D.J. and Rachel R. “The outer membrane of the hyperthermophilic archaeon Ignicoccus: dynamics, ultrastructure and composition.” Biochemical Society Transactions, 2004, Volume 32, part 2.
(3) Giannone R.J., Heimerl T., Hettich R.L., Huber H., Karpinets T., Keller M., Kueper U., Podar M. and Rachel R. “Proteomic Characterization of Cellular and Molecular Processes that Enable the Nanoarchaeum equitans- Ignicoccus hospitalis Relationship.” PLoS ONE, 2011, Volume 6, Issue 8.
(4) Eisenreich W., Gallenberger M., Huber H., Jahn U., Junglas B., Paper W., Rachel R. and Stetter K.O. “Nanoarchaeum equitans and Ignicoccus hospitalis: New Insights into a Unique, Intimate Association of Two Archaea.” Journal of Bacteriology, 2008, DOI: 10.1128/JB.01731-07.
(5) Grosjean E., Huber H., Jahn U., Sturt H, and Summons R. “Composition of the lipids of Nanoarchaeum equitans and their origin from its host Ignicoccus sp. strain KIN4/I.” Arch Microbiol, 2004, DOI: 10.1007/s00203-004-0725-x.
(6) Briegel A., Burghardt T., Huber H., Junglas B., Rachel R., Walther P. and Wirth R. “Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell–cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography.” Arch Microbiol, 2008, DOI 10.1007/s00203-008-0402-6.
(7) Burghardt T., Huber H., Junglas B., Naether D.J. and Rachel R. “The dominating outer membrane protein of the hyperthermophilic Archaeum Ignicoccus hospitalis: a novel pore-forming complex.” Molecular Microbiology, 2007, Volume 63.
(8) Berg I.A., Eisenreich W., Eylert E., Fuchs G., Gallenberger M., Huber H.,Jahn U. and Kockelkorn D. “A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis.” PNAS, 2008, Volume 105, issue 22.
(9) Brochier C., Gribaldo S., Zivanovic Y., Confalonieri F. and Forterre P. “Nanoarchaea: representatives of a novel archaeal phylum or a fast-evolving euryarchaeal lineage related to Thermococcales?” Genome Biology 2005, DOI:10.1186/gb-2005-6-5-r42.
(10) Huber H., Rachel R., Riehl S. and Wyschkony I. “The ultrastructure of Ignicoccus: Evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon.” Archaea, 2002, Volume 1.