Helicoverpa armigera densovirus 1: Difference between revisions
| Line 27: | Line 27: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
HaDNV-1 is a novel member of the genus ''Iteradensovirus''. It has an icosahedral structure ranging from 18-26nm in diameter and is a part of the | HaDNV-1 is a novel member of the genus ''Iteradensovirus''. It has an icosahedral structure ranging from 18-26nm in diameter and is a part of the nonenveloped viruses. | ||
The metabolism of ''H. armigera'' includes utilizing the Cotton Bollworm larvae's metabolic processes, specifically aerobic respiration, by benefiting from the metabolites and energy sources produced. HaDNV-1 benefiting from ''H. armigera'' metabolism suggests a mutualistic symbiosis where HaDNV-1 gets to use the product metabolites and the cotton bollworm larvae receive the developmental benefits. | The metabolism of ''H. armigera'' includes utilizing the Cotton Bollworm larvae's metabolic processes, specifically aerobic respiration, by benefiting from the metabolites and energy sources produced. HaDNV-1 benefiting from ''H. armigera'' metabolism suggests a mutualistic symbiosis where HaDNV-1 gets to use the product metabolites and the cotton bollworm larvae receive the developmental benefits. | ||
Latest revision as of 00:03, 26 April 2022
Classification
Higher order Taxa
Viruses (+ ssDNA virus); Shotokuvirae; Cossaviricota; Quintoviricetes; Piccovirales; Parvoviridae; Densovirus; Iteradensovirus
Species
|
NCBI: Helicoverpa armigera densovirus 1 Taxonomy [1] |
Helicoverpa armigera densovirus (genus), HaDNV1 (species)
Helicoverpa armigera densovirus (HaDNV-1) is a single stranded DNA virus that exists in a mutualistic symbiosis with the cotton bollworm, H. armigera. A non-infected strain of H. armigera is a crop pest that feeds on agricultural resources, such as cotton, corn, rice, tomato, chickpea, and many other crops. An infected strain of the cotton bollworm does the same, however at a greater effect. The densovirus directly increases the early growth rate of H. armigera which results in the increase of overall fitness of the organism. In addition, densoviruses protect H. armigera from biopesticides like Baculovirus (Bt) toxin. This protection allows the cotton bollworm to survive the biopesticide efforts and continue to feed on crops. This polyphagous, migratory species can be found in a number of areas, with its native home being Australia and Oceania, it can also be found in Brazil and the Caribbean. Since H. armigera depends on crops for survival, its habitat is based on largely horticultural areas where it can feed on more than 120 plant species in the appropriate climate.
Genome Structure
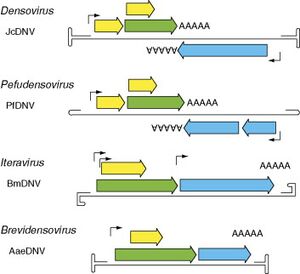
Helicoverpa armigera densovirus is a small icosahedral virus with a genome ranging from 4 to 6 kilobases in size. However, the size and organization of densovirus vary and given their structure, they can be classified into three different groups. The first group corresponds to densoviruses, like HaDNV-1 that have inverted terminal repeats on the DNA strand. Additionally, these viruses have an ambisense structure, meaning that both nonstructural (NS) and structural (VP) proteins are found on the starting 5' end of the DNA strand. On the other hand, viruses that belong to the Iteravirus or Brevidensovirus are monosense which suggests that the NS and VP proteins can be found at both the starting 5' end and the 3' end of the DNA strand.
Cell Structure, Metabolism and Life Cycle
HaDNV-1 is a novel member of the genus Iteradensovirus. It has an icosahedral structure ranging from 18-26nm in diameter and is a part of the nonenveloped viruses.
The metabolism of H. armigera includes utilizing the Cotton Bollworm larvae's metabolic processes, specifically aerobic respiration, by benefiting from the metabolites and energy sources produced. HaDNV-1 benefiting from H. armigera metabolism suggests a mutualistic symbiosis where HaDNV-1 gets to use the product metabolites and the cotton bollworm larvae receive the developmental benefits.
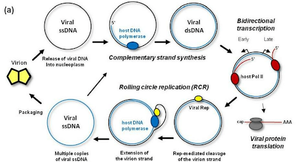
The life cycle of the virus starts with a viron attaching itself to a host membrane of cells in the S phase, where it disassembles and enters the host membrane by endocytosis. The genomic DNA then undergoes a complementary strand synthesis followed by bilateral transcription which runs in two different directions. The viral proteins are translated, undergo rolling replication for repackaging, and finally sent off to infect the neighboring cells.
HaDNV-1 was found in adult female cotton bollworm moths, however, their significance is unknown. One hypothesis suggests that the adult female cotton bollworm moth passes its mutualistic symbiont to its offspring where it can reap the benefits of the symbiosis.
Ecology and Pathogenesis

The Helicoverpa armigera densovirus HaDNV-1 (HaDNV-1) lives within its mutualist symbiont, the cotton bollworm moth (Helicoverpa armigera) larvae and pupa. H. armigera resides on the cotton plant and other crops throughout Asia, Africa, and Australasia. HaDNV-1 provides the moth larvae and pupa with an increased development rate, adult female longevity and fecundity, and protects the larvae from the baculovirus biopesticide, Helicoverpa armigera nucleopolyhedrovirus (HaNPV). HaDNV-1 possibly aids in H. armigera's tolerance of the baculovirus Cry1Ac toxin in bollworm strains that are susceptible to biopesticide. Cry1Ac is responsible for forming pores in the cell membrane by interacting with APN and ABC transporters on the cell membrane surface. The pores formed ultimately result in the death of the insect. However, larval resistance to the Cry1Ac toxin suggests that HaDNV-1 inhibits the binding of the toxin and ABC transporter to protect the host. This saves the larvae and pupa from death. As a consequence, farmers are now trying to utilize new techniques to limit bollworm moth infestation of various plants due to this rising resistance to HaNPV biopesticides. The newly discovered symbiosis of HaDNV-1 and H. armigera poses new threats to the cotton industry.
References
4. Ecosostenibile. (2021, June 30). (Italiano) helicoverpa armigera: Sistematica, habitat, Ciclo Biologico, lotta ... Un Mondo Ecosostenibile. Retrieved April 25, 2022, from https://antropocene.it/en/2021/06/30/helicoverpa-armigera/
5. ScienceDirect. (2016). Densovirus. Densovirus - an overview | ScienceDirect Topics. Retrieved April 25, 2022, from https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/densovirus#:~:text=Densoviruses%20(DNVs)%20are%20small%20icosahedral,counterparts%2C%20in%20the%20family%20Parvoviridae
Author
Page authored by Abigael Frederick, David Duncan, and Bradley Enneking, students of Prof. Jay Lennon at Indiana University.