Herpesviridae: Viral Cycle, Capsid Transport, and Cancer Treatment
Introduction
By Michael Gallaher
Herpes is a family of DNA viruses called Herpesviridae. The herpes family of viruses consists of three families of viruses; Alphaherpesviridae, Betaherpesviridae, and Gammaherpesviridae. Among these families are many commonly known viruses that are causative agents of many diseases, such as HSV-1 and HSV-2 for cold sores and genital warts, varicella zoster for chicken pox and shingles, and the Eppstein-Barr virus for mononucleosis. Herpes virsues also tend to have latent, recurring infections in the infected organisms, where the virus remains in some part of the infected organism (Gupta, 2007). Herpes family viruses are incredibly common, with at least 90% of people within the United States having been infected with at least one form of Herpesviridae (CDC, 2006).
Of these viral families, the animal herpes viruses that cause disease are of the Alphaherpesviridae family. The most well known examples of herpes are the herpes simplex viruses, HSV-1 and HSV-2. HSV-1 is the causative pathogen of most oral cold sores, while HSV-2 is the causative agent of genital warts. It is thought that more than 50% of adults in the United States have HSV-1, and that nearly 20% have HSV-2 (CDC, 2010). The widespread infection of the populace makes herpes a good target for medical research. Another promising field of research that has developed more recently is the possibility of using a re-engineered HSV as a treatment vector for other diseases, such as various forms of cancer (Varghese, 2002).
Pathology
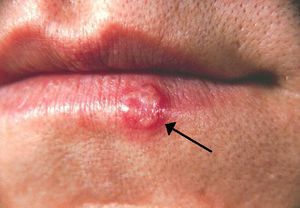
HSV-1 and HSV-2 are both transmitted through skin-to-skin contact or through contact with an infected person’s bodily fluids. HSV-1 primarily infects the areas of the mouth, throat, face, eyes, and the central nervous system, while HSV-2 primarily infects the genitals. However, both variants can infect all areas (Gupta, 2007). Transmission can also be vertical through pregnancy, and neonatal HSV infection is more severe than childhood or adult. Once infected, HSV causes blisters on the skin that are filled with active virus particles. Contact with these blisters carries the highest risk of transmission. However, these blisters are not permanent, lasting typically a few weeks. The infection then goes into a latent stage, where the infected person is asymptomatic. Though asymptomatic, the infected person may still transmit the virus. During the latent stage, HSV invades peripheral neurons and remains in the nuclei of these cells (Gupta, 2007). Eventually, the virus is triggered and re-infection begins, as virions move out of neuronal nuclei and out to re-infect epithelial areas. It is unknown exactly what triggers the resurgence of the virus. Interestingly, once infected other areas become resistant to infection: If an oral infection of HSV-1 occurs, then infections will not occur on the fingers (Gupta, 2007). Also, contracting one variant confers some protection against the other, as those infected with HSV-1 are somewhat resistant to HSV-2, while those infected with HSV-2 are resistant to HSV-1 (Gupta, 2007). Regardless of which strain is contracted, the immune system never eradicates the infection. Re-activations of the virus often become less severe and more infrequent over time, with some infected individuals becoming completely asymptomatic (Gupta, 2007).
Structure and Genomics
Virion Structure:
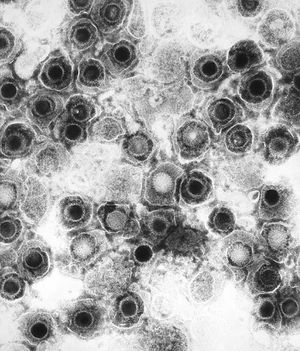
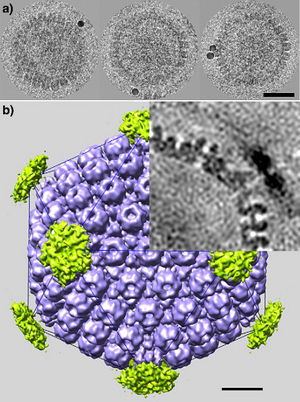
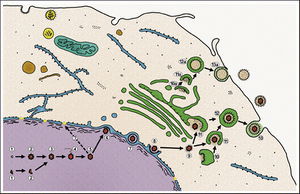
All animal herpes viruses share a similar virion structure, consisting of a protein capsid containing the viral DNA that is surrounded by a membrane envelope that is connected to the capsid by a protein tegument (Mettenleiter, 2006). HSV is a DNA based virus; it has a linear, double stranded genome. The protein capsid is in an icosahedral shape approximately 15 nm thick and 125 nm in diameter (Newcomb et al). There are five conserved proteins that comprise herpes capsids: UL19, which is the major capsid protein that is in all herpes capsids, UL18 and UL38, which are proteins that form triplexes that interact with and help to stabilize adjacent capsids, UL35, which covers all hexons, and UL6, which forms the portal through which the viral genome is injected into the nucleus. The capsid is connected to the membrane envelope via the viral tegument, with which these capsid proteins associate (Mettenleiter, 2006). Herpes virions have also been found to contain cellular proteins, including cytoskeletal components actin and tubulin, heat shock proteins Hsp70 and Hsp90, and annexin. However, it is unknown exactly what role these cellular proteins play and why they are recruited during replication of virions. These cellular proteins are abundant in infected cells during virion replication, and so it is uncertain if these proteins are randomly recruited as “filler material”, or whether they serve a purpose such as priming newly infected cells for synthesis of virion components (Mettenleiter, 2006).
HSV genomics:
The HSV genome is a relatively long genome, with HSV-1 and HSV-2 each encoding at least 74 genes (McGeoch et al, 2006). The herpes genome is linear, and comprised of two main regions, the unique long region (UL), and the unique short region (US). The long region contains 56 genes, while the short region contains 12 genes. Genes in both regions encode for all of the necessary structural components of the virus, including capsid, tegument, and envelope proteins, as well as genes that control viral replication processes and infective ability. Herpes utilizes the RNA Polymerase II of the infected host to transcribe its genes (McGeoch et al, 2006). The genes can be classified into immediate-early, early, and late viral genes. Immediate-early genes encode for the transcription of proteins that regulate the expression early and late genes, and these proteins are also part of the tegument that enters the cell after the capsid (McGeoch et al, 2006). Early genes are expressed after the immediate-early genes, and these genes control the biosynthesis of enzymes involved in DNA replication, as well as the production of various glycoproteins that comprise the virion envelope. The late genes primarily encode for the proteins that form the capsid and tegument and form the virion particle (McGeoch et al, 2006).
Section 3
Include some current research, with at least one figure showing data.
Conclusion
Overall text length at least 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.
