Hyperthermophilic archaeal cellular structures: Difference between revisions
Emannucc0295 (talk | contribs) No edit summary |
Emannucc0295 (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
[http://en.wikipedia.org/wiki/Hyperthermophile | [http://en.wikipedia.org/wiki/Hyperthermophile Hyperthermophiles] are organisms that can live at temperatures ranging between 80-122ºC. They have been the subject of intense study since their discovery in 1977 in the [http://en.wikipedia.org/wiki/Gal%C3%A1pagos_hotspot Galapagos Rift]<sup>1</sup>. It was thought impossible for life to exist at temperatures a great as 100ºC until [https://microbewiki.kenyon.edu/index.php/Pyrolobus_fumarii <i>Pyrolobus fumarii</i>] was discovered in 1997<sup>2</sup>. <i>P. fumarii</i> is an unicellular organism from the [http://en.wikipedia.org/wiki/Domain_(biology) domain] [http://en.wikipedia.org/wiki/Archaea Archaea] living in the [http://en.wikipedia.org/wiki/Hydrothermal_vent hydrothermal vents] in [http://en.wikipedia.org/wiki/Black_smoker#Black_smokers_and_white_smokers black smokers] along the [http://en.wikipedia.org/wiki/Mid-Atlantic_Ridge Mid-Atlantic Ridge]<sup>2</sup>. These organisms can live at 106ºC at a pH of 5.5<sup>2</sup>. In order to get energy from their environment these organisms are facultatively [http://en.wikipedia.org/wiki/Aerobic_organism aerobic] obligate chemolithoautotrophs, meaning these organisms build biomolecules by harvesting CO<sub>2</sub> from their environment by using H<sub>2</sub> as their primary electron donor and NO<sub>3</sub><sup>-</sup> as its primary electron acceptor<sup>2</sup>. These organisms can even survive the [http://en.wikipedia.org/wiki/Autoclave autoclave], which is a machine designed to kill organisms through high heat and pressure<sup>2</sup>. Because hyperthermophiles live in such hot environments, they need to have [http://en.wikipedia.org/wiki/DNA DNA], [http://en.wikipedia.org/wiki/Membrane membrane ]and [http://en.wikipedia.org/wiki/Enzyme enzyme] modifications in order to withstand the intense thermal energy. Such modifications are currently being studied to better understand what allows an organism or protein to survive such harsh conditions. By learning what allows these organisms to survive such harsh conditions, researchers will be better able to synthesize molecules that are harder to denature that can be used in industry. | ||
==DNA structures of <i>P. fumarii</i>== | ==DNA structures of <i>P. fumarii</i>== | ||
Two DNA strands are held together by [http://en.wikipedia.org/wiki/Base_pair base pairing] that allows the [http://en.wikipedia.org/wiki/Nucleotide nucleotide bases] [http://en.wikipedia.org/wiki/Adenosine adenosine] (A) to bind with [http://en.wikipedia.org/wiki/Thymine thymine] (T), and [http://en.wikipedia.org/wiki/Guanine guanine] (G) to bind with [http://en.wikipedia.org/wiki/Nucleotide cytosine] (C). It has been proposed that thermophilic archaea would be expected to have higher GC content within their DNA, because GC pairings have three [http://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonds], while AT pairings have only two. Increasing the number of hydrogen bonds would increase the stability of the DNA, thereby increasing the energy required to separate the two strands of DNA. This would help the DNA to remain double stranded while at such high temperatures that would normally provide enough thermal energy to separate the DNA strands.3 | |||
<i>P. fumarii</i> was first sequenced in 2001 by the Diversa Corporation and the sequence was released to the public in 20114. The data from this analysis showed a GC content of 54.90%. This supports the hypothesis that thermophiles experience selective pressure to increase their GC content in order to stabilize their DNA5. However, research has not conclusively supported this hypothesis. A study done by Hurst and Merchant (2001) showed no correlation between higher GC content in prokaryotes and increased optimal growing temperatures. However, their analysis did show that there was higher GC content for the third [http://en.wikipedia.org/wiki/Amino_acid amino acid] within the [http://en.wikipedia.org/wiki/Codon codon]. This demonstrates that within the [http://en.wikipedia.org/wiki/Wobble_position wobble position] there is likely a selective pressure for more hydrogen bonds to increase stability within the DNA, but less selective pressure for GC pairings within the DNA as a whole5. This supports what is seen in <i>P. fumarii</i>. The majority of the DNA is composed of G and C nucleotides, but the DNA still contains many A and T nucleotides. These results likely indicate that along with increasing GC pairing in the wobble position, thermophilic archaea have other mechanisms for stabilizing their DNA at such high temperatures5. | |||
One possible mechanism for stabilizing DNA at such high temperatures are proteins such as a type I [http://en.wikipedia.org/wiki/Topoisomerase topoisomerase] that supertwists the DNA making spontaneously untwisting of the DNA more difficult. The presence of this protein in multiple evolutionarily distant organisms supports the hypothesis that this protein plays a role in DNA stabilization.6 | |||
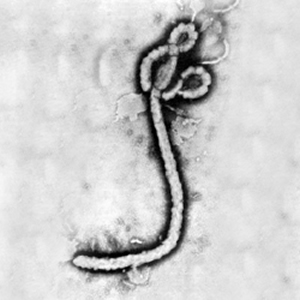
[[Image:Ebola virus 1.jpeg|thumb|300px|right|Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.]] | [[Image:Ebola virus 1.jpeg|thumb|300px|right|Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.]] | ||
| Line 14: | Line 20: | ||
<br><b>Legend/credit:</b> Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC. | <br><b>Legend/credit:</b> Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC. | ||
<br><b>Closed double brackets:</b> ]] | <br><b>Closed double brackets:</b> ]] | ||
Revision as of 06:07, 21 March 2014
Hyperthermophiles are organisms that can live at temperatures ranging between 80-122ºC. They have been the subject of intense study since their discovery in 1977 in the Galapagos Rift1. It was thought impossible for life to exist at temperatures a great as 100ºC until Pyrolobus fumarii was discovered in 19972. P. fumarii is an unicellular organism from the domain Archaea living in the hydrothermal vents in black smokers along the Mid-Atlantic Ridge2. These organisms can live at 106ºC at a pH of 5.52. In order to get energy from their environment these organisms are facultatively aerobic obligate chemolithoautotrophs, meaning these organisms build biomolecules by harvesting CO2 from their environment by using H2 as their primary electron donor and NO3- as its primary electron acceptor2. These organisms can even survive the autoclave, which is a machine designed to kill organisms through high heat and pressure2. Because hyperthermophiles live in such hot environments, they need to have DNA, membrane and enzyme modifications in order to withstand the intense thermal energy. Such modifications are currently being studied to better understand what allows an organism or protein to survive such harsh conditions. By learning what allows these organisms to survive such harsh conditions, researchers will be better able to synthesize molecules that are harder to denature that can be used in industry.
DNA structures of P. fumarii
Two DNA strands are held together by base pairing that allows the nucleotide bases adenosine (A) to bind with thymine (T), and guanine (G) to bind with cytosine (C). It has been proposed that thermophilic archaea would be expected to have higher GC content within their DNA, because GC pairings have three hydrogen bonds, while AT pairings have only two. Increasing the number of hydrogen bonds would increase the stability of the DNA, thereby increasing the energy required to separate the two strands of DNA. This would help the DNA to remain double stranded while at such high temperatures that would normally provide enough thermal energy to separate the DNA strands.3
P. fumarii was first sequenced in 2001 by the Diversa Corporation and the sequence was released to the public in 20114. The data from this analysis showed a GC content of 54.90%. This supports the hypothesis that thermophiles experience selective pressure to increase their GC content in order to stabilize their DNA5. However, research has not conclusively supported this hypothesis. A study done by Hurst and Merchant (2001) showed no correlation between higher GC content in prokaryotes and increased optimal growing temperatures. However, their analysis did show that there was higher GC content for the third amino acid within the codon. This demonstrates that within the wobble position there is likely a selective pressure for more hydrogen bonds to increase stability within the DNA, but less selective pressure for GC pairings within the DNA as a whole5. This supports what is seen in P. fumarii. The majority of the DNA is composed of G and C nucleotides, but the DNA still contains many A and T nucleotides. These results likely indicate that along with increasing GC pairing in the wobble position, thermophilic archaea have other mechanisms for stabilizing their DNA at such high temperatures5.
One possible mechanism for stabilizing DNA at such high temperatures are proteins such as a type I topoisomerase that supertwists the DNA making spontaneously untwisting of the DNA more difficult. The presence of this protein in multiple evolutionarily distant organisms supports the hypothesis that this protein plays a role in DNA stabilization.6
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: Ebola virus 1.jpeg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Overall paper length should be 3,000 words, with at least 3 figures with data.
Section 2
Include some current research in each topic, with at least one figure showing data.
Section 3
Include some current research in each topic, with at least one figure showing data.
Further Reading
[Sample link] Ebola Hemorrhagic Fever—Centers for Disease Control and Prevention, Special Pathogens Branch
References
Edited by Libby Mannucci, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.