Jack's wip page: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
<br>While wild animals are more frequently the source of zoonoses, the animals we have domesticated have their own virulent and devastating diseases <ref name=Qiu/> | <br>While wild animals are more frequently the source of zoonoses, the animals we have domesticated have their own virulent and devastating diseases <ref name=Qiu/> | ||
<ref name= Minardi>[https://www.mdpi.com/1999-4915/5/7/1867 Minardi da Cruz, et al., "Small Ruminant Lentiviruses (SRLVs) Break the Species Barrier to Acquire New Host Range" 2013. Viruses 5:1867-1884.]</ref>. In addition, these livestock pathogens also cause issues for people because of the significant economic losses they can inflict <ref name=Kalogianni>[https://www.mdpi.com/2076-2615/10/4/616 Kalogianni et al.: Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. 2020. Animals 10:616.]</ref> <ref name=Chengula>[https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-549 Chengula et al.: "Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania" 2013. SpringPlus 2:549.]</ref>. This problem is exacerbated when the causative pathogens have no vaccine, or treatment, or are under-researched. This is the case for many neglected tropical diseases (NTD) like Rift Valley Fever virus ( | <ref name= Minardi>[https://www.mdpi.com/1999-4915/5/7/1867 Minardi da Cruz, et al., "Small Ruminant Lentiviruses (SRLVs) Break the Species Barrier to Acquire New Host Range" 2013. Viruses 5:1867-1884.]</ref>. In addition, these livestock pathogens also cause issues for people because of the significant economic losses they can inflict <ref name=Kalogianni>[https://www.mdpi.com/2076-2615/10/4/616 Kalogianni et al.: Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. 2020. Animals 10:616.]</ref> <ref name=Chengula>[https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-549 Chengula et al.: "Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania" 2013. SpringPlus 2:549.]</ref>. This problem is exacerbated when the causative pathogens have no vaccine, or treatment, or are under-researched. This is the case for many neglected tropical diseases (NTD) like Rift Valley Fever virus (RVFV) as well as others like Maedi-Visna virus (MVV). This often gives the | ||
<br> | <br> | ||
==Rift Valley Fever== | ==Rift Valley Fever== | ||
[[Image: RVFV.jpg |thumb|250px|left| <i>Phlebovirus</i> responsible for Rift Valley Fever. Photo credit: https://thenativeantigencompany.com/products/rift-valley-fever-virus-nucleoprotein/]] | |||
< | |||
===Virus=== | ===Virus=== | ||
Rift Valley Fever is caused by an approximately 100 nm diameter spherical <i>Phlebovirus</i> in the family <i>Bunyaviridae</i>. It has a negative sense RNA genome with three segments, L, M, and S. The large (L) segment codes for the virus’s RNA-dependent RNA polymerase, and the medium (M) segment codes for the glycoproteins that coat the surface of the viral lipid bilayer envelope (Pepin). The small (S) segment is ambisense and codes for two proteins: a nucleoprotein and a virulence factor (Lorenzo). The nucleoprotein (N) aids the RNA-dependent RNA polymerase in transcription and replication of the viral genome. The virulence factor (NSs) plays a significant role in suppressing the innate host immune response (Lorenzo). The NSs factor suppresses the immune system by forming fibrils, clusters of the NSs proteins that appear filamented, which act as a general inhibitor of transcription as well as induce many DNA damage signaling proteins within the cell (Baer). This has the effect of halting the cell cycle, typically in S phase, which allows for greater viral replication (Lorenzo, Baer).<br> | |||
<br> The virus is also an arthropod-borne virus or arbovirus (CDC). For RVF, the virus is vectored by the species in the <i>Aedes</i> and <i>Culex</i> genus. This transmission varies on location but is often spread by the <i>Aedes aegypti</i> mosquito due to the near-global distribution. Additionally, RVFV can be transmitted through the eggs of <i>Aedes aegypti</i> mosquitoes (CDC, Lorenzo). This vertical transmission of the virus from a female mosquito to her offspring plays a significant role in the persistence of the virus. In this way, the mosquito acts not just as a vector of the virus, but also as a reservoir host and can maintain the presence of the virus in the absence of mammals to infect. Additionally, when there are floods, and female Aedes mosquitoes have more opportunities for egg-laying, there are many more cases of RVF in animal and human populations because there is an influx of RVFV positive mosquitoes (CDC). The virus can also be spread between animals in contaminated body fluids. These two modes of transmission, by mosquito vector and bodily fluid exposure, contribute to human exposure to the virus. | |||
===Animal Health=== | ===Animal Health=== | ||
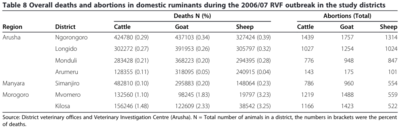
[[Image: RVF-2007-death-abortions.png |thumb|400px|right| Deaths and abortions caused by RVF in the 2006/2007 outbreak in Tanzania. Photo credit: <ref name=Chengula/> https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-549]] | |||
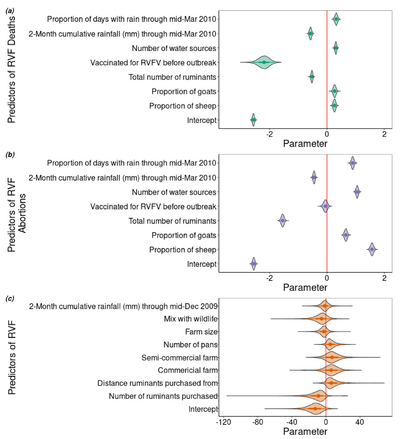
[[Image: Predictors of RVF.png |thumb|400px|right| Factors that predict RVF deaths, abortions, and infection during 2010 Rift Valley Outbreak in Central South Africa. Photo Credit : Rostal. et al., 2020]] | |||
===Economic Impact=== | ===Economic Impact=== | ||
| Line 61: | Line 43: | ||
==Conclusion== | ==Conclusion== | ||
<br>At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.<br><br>The insertion code consists of: | |||
<br><b>Double brackets:</b> [[ | |||
<br><b>Filename:</b> PHIL_1181_lores.jpg | |||
<br><b>Thumbnail status:</b> |thumb| | |||
<br><b>Pixel size:</b> |300px| | |||
<br><b>Placement on page:</b> |right| | |||
<br><b>Legend/credit:</b> Magnified 20,000X, this colorized scanning electron micrograph (SEM) depicts a grouping of methicillin resistant <i>Staphylococcus aureus</i> (MRSA) bacteria. Photo credit: [http://www.cdc.gov/ CDC.] Every image requires a link to the source. | |||
<br><b>Closed double brackets:</b> ]] | |||
<br><br>Other examples: | |||
<br><b>Bold</b> | |||
<br><i>Italic</i> | |||
<br><b>Subscript:</b> H<sub>2</sub>O | |||
<br><b>Superscript:</b> Fe<sup>3+</sup> | |||
<br> | |||
<br> | |||
Sample citations: <ref name=aa>[http://www.plosbiology.org/article/fetchObject.action?uri=info%3Adoi%2F10.1371%2Fjournal.pbio.1000005&representation=PDF Hodgkin, J. and Partridge, F.A. "<i>Caenorhabditis elegans</i> meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.]</ref> | |||
<ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847443/ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.]</ref> | |||
<br><br>A citation code consists of a hyperlinked reference within "ref" begin and end codes. | |||
<br>To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>" | |||
<br> The repeated citation works like this, with a forward slash.<ref name=aa/> | |||
==References== | ==References== | ||
<references /> | <references /> | ||
<br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | <br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | ||
Latest revision as of 19:15, 14 April 2024
Introduction
By Jack Caine
For thousands of years humans have cultivated animals as companions and food sources. This long-lasting and close relationship, in addition to providing a foundation for human global expansion, has exposed us to many of their pathogens [1]. While these diseases can be quite deadly in their original hosts, the opportunity for a pathogen to be transmitted to a human from an animal increases with duration with them. Zoonoses are any disease that originates in animals and is transmitted to humans [2]. Nearly 60% of known infectious diseases come from zoonotic sources [1]. While zoonoses are not the most common type of disease in humans they do have the capacity to have outbreaks which can result in epidemics or pandemics. Many of the world’s most recent and devastating epidemics and pandemics have resulted from the spillover--the introduction of a novel pathogen into humans from animals--of diseases previously only found in animals. The most well-known of these are the sudden acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics, the SARS-CoV-2 pandemic, and the ongoing AIDS pandemics [1].
While wild animals are more frequently the source of zoonoses, the animals we have domesticated have their own virulent and devastating diseases [1]
[3]. In addition, these livestock pathogens also cause issues for people because of the significant economic losses they can inflict [4] [5]. This problem is exacerbated when the causative pathogens have no vaccine, or treatment, or are under-researched. This is the case for many neglected tropical diseases (NTD) like Rift Valley Fever virus (RVFV) as well as others like Maedi-Visna virus (MVV). This often gives the
Rift Valley Fever

Virus
Rift Valley Fever is caused by an approximately 100 nm diameter spherical Phlebovirus in the family Bunyaviridae. It has a negative sense RNA genome with three segments, L, M, and S. The large (L) segment codes for the virus’s RNA-dependent RNA polymerase, and the medium (M) segment codes for the glycoproteins that coat the surface of the viral lipid bilayer envelope (Pepin). The small (S) segment is ambisense and codes for two proteins: a nucleoprotein and a virulence factor (Lorenzo). The nucleoprotein (N) aids the RNA-dependent RNA polymerase in transcription and replication of the viral genome. The virulence factor (NSs) plays a significant role in suppressing the innate host immune response (Lorenzo). The NSs factor suppresses the immune system by forming fibrils, clusters of the NSs proteins that appear filamented, which act as a general inhibitor of transcription as well as induce many DNA damage signaling proteins within the cell (Baer). This has the effect of halting the cell cycle, typically in S phase, which allows for greater viral replication (Lorenzo, Baer).
The virus is also an arthropod-borne virus or arbovirus (CDC). For RVF, the virus is vectored by the species in the Aedes and Culex genus. This transmission varies on location but is often spread by the Aedes aegypti mosquito due to the near-global distribution. Additionally, RVFV can be transmitted through the eggs of Aedes aegypti mosquitoes (CDC, Lorenzo). This vertical transmission of the virus from a female mosquito to her offspring plays a significant role in the persistence of the virus. In this way, the mosquito acts not just as a vector of the virus, but also as a reservoir host and can maintain the presence of the virus in the absence of mammals to infect. Additionally, when there are floods, and female Aedes mosquitoes have more opportunities for egg-laying, there are many more cases of RVF in animal and human populations because there is an influx of RVFV positive mosquitoes (CDC). The virus can also be spread between animals in contaminated body fluids. These two modes of transmission, by mosquito vector and bodily fluid exposure, contribute to human exposure to the virus.
Animal Health
Economic Impact
Human Health
Every point of information REQUIRES CITATION using the citation tool shown above.
Maedi-Visna
Include some current research, with at least one figure showing data.
Virus
Animal Health
Economic Impact
Human Health
Solutions
Include some current research, with at least one figure showing data.
Section 4
Conclusion
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Magnified 20,000X, this colorized scanning electron micrograph (SEM) depicts a grouping of methicillin resistant Staphylococcus aureus (MRSA) bacteria. Photo credit: CDC. Every image requires a link to the source.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Sample citations: [6]
[7]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>"
The repeated citation works like this, with a forward slash.[6]
References
- ↑ 1.0 1.1 1.2 1.3 Qiu, Y. et al., "Global prioritization of endemic zoonotic diseases for conducting surveillance in domestic animals to protect public health." 2023. Phil. Trans. R. Soc. B 378.
- ↑ Quammen, David. "Spillover: animal infections and the next human pandemic." 2012. W.W. Norton & Company.
- ↑ Minardi da Cruz, et al., "Small Ruminant Lentiviruses (SRLVs) Break the Species Barrier to Acquire New Host Range" 2013. Viruses 5:1867-1884.
- ↑ Kalogianni et al.: Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. 2020. Animals 10:616.
- ↑ 5.0 5.1 Chengula et al.: "Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania" 2013. SpringPlus 2:549.
- ↑ 6.0 6.1 Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024