Junin virus
1. Introduction
The Argentinian Mammarenavirus, more commonly known as the Junin Virus, is a strain of the New World Mammarena Virus found specifically in Argentina[2] . It primarily affects farmworkers in the pampas region of Argentina [3], [4]. While early-stage infection presents with flu-like symptoms, late-stage infection can cause Argentine Hemorrhagic Fever, a potentially lethal condition characterized by system-wide hemorrhagic bleeding[2]. Junin Virus has related strains all over the world, a well-known one being the Lassa Virus in West Africa[5]. While some treatments and vaccines have proven effective in stemming some of its spread, Junin Virus continues to make its way throughout the region. It spreads through contact with bodily fluids, aerosolization, and contact with contaminated materials (medical equipment for example)[2]. Mammarenaviruses as a whole were left understudied for a long time due to their presence mainly being in underdeveloped and underserved regions, leaving a gap in the medical research that is only now beginning to be filled.
Classification of Junin Virus
- Superkingdom
- Viruses
- Domain
- Riboviria
- Kingdom
- Orthonavirae
- Phylum
- Negarnviricota
- Subphylum
- Polyploviricotina
- Class
- Ellioviricetes
- Order
- Bunyavirales
- Genus
- Arenaviridae
- Species
- Mammarenavirus
- Common Names
- Argentine Mammarenavirus, Junin Virus, Junin Arenavirus, JUNV
2. Ecology
The Junin Virus, also known as “Argentine Mammarenavirus” is mainly found in central Argentina where the majority of farming occurs[2]. This is because it is a virus contracted from Calomys musculinus mice native to this region[2]. As a result of the frequent contact with these rodents, mostly farmers are impacted by this virus and it first arose through a jump from mice to humans in the 1950s[2]. It also can infect other small rodent hosts like rabbits and guinea pigs[2]. These infections are caused by multiple different strains of Junin Virus of multiple different virulences. The most virulent strain was dubbed “Romero”. Originally, when Junin Virus was first discovered, since it led to hemorrhagic fever, the fatality rate was higher(around 16.5%, however once the vaccine was introduced, fatality decreased exponentially[2]. Scientifically the Junin Virus is classified as a biosafety level four pathogen meaning that it is highly virulent and if handled in a lab environment requires extreme precautions [23].
Junin virus requires a pH less than 6.1 to enter the host cell membrane via endocytosis [9] but is inactivated at pH below 5.5 or above 8.5 [21,22]. The virus is also sensitive to heat and inactivated if exposed to a temperature of 56°C for at least 30 minutes. Junin virus is also inactivated by gamma irradiation and UV radiation [21,22].
3. Viral Structure
Junin Virus is coated in a viral envelope consisting of a phospholipid bilayer and glycoproteins. Its shape can be pleomorphic (meaning shapes can vary) or spherical[2]. It has a diameter of 110-130 nm and has several glycoprotein spikes embedded in the lipid bilayer that are each 8-10 nm long[2]. These spikes are made up of the glycoproteins GP1 and GP2, which are produced by cleaving the glycoprotein precursor (GPC), a protein that is encoded in the viral genome[2]. The glycoprotein spike is club-shaped, with the head of the club consisting of a GP1 tetramer and the stem consisting of a GP2 tetramer[2]. The nucleoprotein (NP) is another protein encoded in the viral genome, and it associates with the viral RNA to form nucleocapsid structures in the cytoplasm of the infected cell[2]. The lipid membrane of the virus has its origin in the host cell membrane, and packaged inside are ribosomes also derived from the host cell[2]. It is currently unknown if the ribosomes serve any purpose[2].
4. Viral Replication
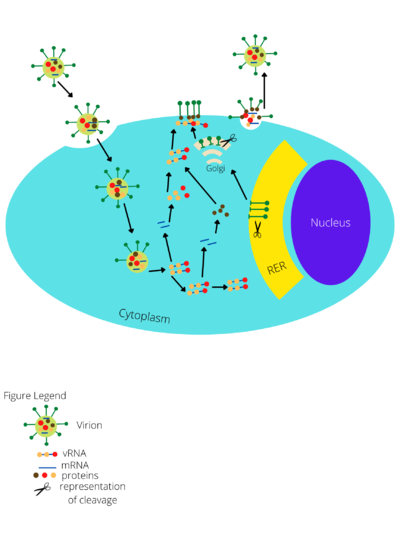
Cell Entry
Junin Virus enters cells via clathrin-mediated endocytosis (Figure 1)[9]. Internalization of the Junin Virus involves the formation of clathrin-coated pits (CCPs), which are infoldings of the host cell membrane that are formed after the virus attaches to a plasma membrane receptor[9]. A clathrin-coated vesicle (CCV) (also known as an endosome) is then pinched off from the inside face of the cell membrane and taken into the cell[7]. After being internalized, the viral membrane fuses with the vesicle membrane, releasing the virions in the cytoplasm. This process of fusion is dependent on acidic conditions in the endosome[9]. The virus fuses with the vesicle membrane when the endosome has a pH of less than 6.1[9]. It is not entirely clear why a low pH is necessary, but it may be because Junin Virus has external proteins involved in fusion that are structurally altered to perform their function under acidic conditions, similar to Influenza virus, Rubella virus, West Nile virus, and tick-borne encephalitis virus[9].
Replication
The Junin Virus, once it has entered the cell through clathrin-mediated endocytosis, the PI3K/Akt signaling pathway is activated which brings the virus to a more acidic compartment of the endosome[10]. The 3’ and 5’ ends of the viral RNA hybridize and act as a binding site for viral polymerases to transcribe the RNA genome[10]. The NP protein is the first protein that is translated. After translation of GPC, the protein is cleaved into two new glycoproteins: GP1 and GP2. GP1 is inserted in the peripheral membrane of the viral envelope, and GP2 becomes part of the integral membrane[7]. These proteins in the bilayer are involved in recognition and the entry of the virus into target cells. The cleaving of GPC also yields a signal stable peptide (SSP), which ensures that GPC responds properly to acidic conditions[6]. These viral polymerases make full copies of the S and L RNAs and they are capped on the 5’ end but have no 3’-polyA tail[10]. Finally, to terminate transcription, a stem-loop forms[10]. The daughter strands of viral RNA are then packed in virions with other types of RNA. For the final step of replication, the virion is fully assembled. To do this, the N and Z proteins work together to assemble the components of the new virions and initiate viral budding from the host cell membrane with the Z protein being the major player in this process (Figure 1) [10].
5. Genome structure
Junin Virus has a negative-sense double-stranded RNA genome that is made up of two segments: the large (L), which is 7.3 kbp, and the small (S), which is 3.5 kbp[6]. The two segments are configured in an ambisense orientation, which means the information on each strand is read in the opposite direction from the other[6]. The genome of the Junin Virus codes for four proteins. The L segment encodes a viral RNA polymerase called L polymerase, as well as a small zinc-binding protein called RING finger protein Z[6]. The S segment encodes the glycoprotein precursor (GPC) and the nucleoprotein (NP)[6]. The NP protein is the first protein that is translated. After translation of GPC, the protein is cleaved into two new glycoproteins: GP1 and GP2. GP1 is inserted in the peripheral membrane of the viral envelope, and GP2 becomes part of the integral membrane[7]. These proteins in the bilayer are involved in recognition and the entry of the virus into target cells. The cleaving of GPC also yields a signal stable peptide (SSP), which ensures that GPC responds properly to acidic conditions[6].
6. Pathology
Infection of human endothelial cells by the Junin virus increases blood vessel permeability (vascular permeability) to different molecules, which negatively impacts host physiological processes[11]. Upon entry, the virus leads to disorganization of the endothelial cell adherens junctions (ECAJ). Patients who suffer from Argentine Hemorrhagic Fever( AHF )show symptoms such as thrombocytopenia, leukopenia, and fluid distribution problems that are directly related to vascular dysregulation (REF)[11]. Studies show that infection by the Junin Virus increases the production of two important cytokines - IL-6 and MCP-1 that function together during Junin virus infection by regulating endothelial permeability to disrupt cell junctions[11]. The changes in IL-6 and MCP1 levels were only observed in infected cells and cells neighboring infected cells. This indicates that infection with JV leads to initiation of an intracellular cascade that increases signaling of IL-6 and MCP1 cytokines and causes a loss of cadherin junctions leading to subsequent permeability increases.
7. Current Research
Junin Virus research focuses on viral replication machinery, host-virus interactions, treatment, and vaccination. In a human host, the Junin virus has been shown to use its Z protein to hijack human ribosomal proteins, Ras proteins, endosome sorting proteins, and ATP synthesis proteins[12].Another discovery relating to how the Junin Virus interacts with and hijacks human proteins focuses on the virus’ use of the Type 1 Interferon in humans to enter cells [13]. Once inside the cell, this interaction is one of the ways the Junin Virus can cause Argentine Hemorrhagic Fever (AHF)[14]. It was found that when mice were infected with the Junin virus, the mice that had a higher concentration of Type 1 Interferon had more severe AHF symptoms than mice that had a lower concentration[14]. Research into these areas gives the scientific community a more thorough understanding of how JUNV impacts its host and can be used to make more informed decisions on treatment and vaccination against the Junin Virus. Research and discoveries regarding Junin Virus antibodies are also being explored. Experiments being conducted suggest that a glycoprotein present in the Junin Virus vaccine may also be effective in protecting against the Machupo virus, another New World arenavirus[15]. In particular in mice, a recombinant glycoprotein from the Junin Virus strain protected them from the Machupo virus, showing significant reductions in death and infection [16], [17]. These findings allow the possibility of the application of vaccines against more than one virus.
Another emerging area of research is the effect of specific inhibitors on different metabolic pathways of the Junin virus. Two inhibitors, brefeldin A (BFA) and carbonyl cyanide m-chlorophenylhydrazone (CCCP), have been shown to affect the maturation process of the virus by disrupting the virus’s intracellular exocytic pathway [11]. CCCP alters the distribution of glycoproteins in the cell by blocking its transport from the endoplasmic reticulum to the plasma membrane. The disruption in this exocytic pathway leads to an accumulation of JV glycoproteins at the endoplasmic reticulum surface [11]. BFA disrupts the maturation of glycoproteins in the Golgi apparatus GP38 is a glycoprotein responsible for virus attachment to the host cell [11]. Disruption in the transport and maturation of this GP is required for both the maturation and entry of JV into the cell [11], [16]. The combined effect of CCCP and BFA restrict the formation and multiplication of the Junin Virus. Further research in this area is being conducted to gain a better understanding of the complex physiological pathways of the JV and investigate the scope of such inhibitors in the treatment of Argentine Hemorrhagic Fever.
Novel vaccine research has found a potential new way to vaccinate against the Junin Virus that is safer than the current Candid 1 vaccine. The Candid 1 vaccine uses a live-attenuated version of the Junin Virus has, in some cases, been shown to revert to its more virulent form[19]. In light of this potentiality for reversal, the FDA will not approve the Candid 1 vaccine for use in the U.S. A new attenuated version of the Junin virus was developed that was unable to be reverted to its more virulent form, making it a safer option to the Candid 1 vaccine[19]. This new vaccine candidate, when tested in guinea pigs, was able to protect against the Junin Virus while simultaneously not reverting to its original virulent form[19]. Continuing research into safer vaccines for the Junin Virus provides a safer landscape for the citizens of Argentina to live in and contributes to the larger index of information on how vaccines can be made for other viruses.
8. Authorship Statement
This article was authored by Lillian Nielsen, Yufei Lin, Vrinda Kohli, Theodore Morrissey, and Sakshi Shah. Lillian Nielsen was responsible for the introduction and grammar checking of the initial MicrobeWiki Proposal. For the annotated bibliography, she found and annotated a number of journal articles as well as re-read and checked for grammatical errors for the bibliography as well. In the first draft of the wiki article, she was responsible for the classification, introduction, and current research sections as well as grammar checking throughout the draft. Within the current research section, she provided information on how the Junin Virus interacts with its host and novel vaccine research. For the final draft she edited her sections according to peer feedback and checked for grammar errors. Yufei Lin worked on the current research section providing information on Junin Virus antibodies, as well as finding another source for the annotated bibliography. Lillian Nielsen was also responsible for re-reading and checking for grammatical errors in the first and final draft along with Sakshi Shah. Sakshi Shah found primary sources related to the pathogenesis and potential treatments along with checking through all the citations in the paper to make sure they followed the proper guidelines, created the image on the viral life cycle and found information regarding virulence of the Junin Virus. Theodore Morrissey was responsible for the sections on genome structure, virus structure, and cell entry, as well as finding and annotating journal articles for the annotated bibliography. Vrinda Kohli found primary resources related to virus pathology, inhibition and cell entry for the annotated bibliography and worked on the sections of pathology and replication of the virus for the first draft of the article. Lillian Nielsen also helped with the replication section.
9. References
[1]Schoch CL, et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020: baaa062. PubMed: 32761142 PMC: PMC7408187.
[2]Enría, D. A., Mills, J. N., Bausch, D., Shieh, W.-J., & Peters, C. J. 2011. CHAPTER 68—Arenavirus Infections. Tropical Infectious Diseases: Principles, Pathogens, and Practice. Elsevier Inc. Third Edition: 449–461.
[3]Enria, D. A., Briggiler, A. M., & Sánchez, Z. 2008. Treatment of Argentine hemorrhagic fever. Special Issue: Treatment of Highly Pathogenic RNA Viral Infections 78 (1): 132–139.
[4]Maiztegui, J. I., McKee, J., Kelly T., Oro, J. G. B., Harrison, L. H., Gibbs, P. H., Feuillade, M. R., Enria, D. A., Briggiler, A. M., Levis, S. C., Ambrosio, A. M., Halsey, N. A., Peters, C. J., & Group, the A. S. 1998. Protective Efficacy of a Live Attenuated Vaccine against Argentine Hemorrhagic Fever. The Journal of Infectious Diseases, 177 (2): 27–283.
[5]García, J. B., Morzunov, S. P., Levis, S., Rowe, J., Calderón, G., Enría, D., Sabattini, M., Buchmeier, M. J., Bowen, M. D., & Jeor, S. C. S. 2000. Genetic Diversity of the Junin Virus in Argentina: Geographic and Temporal Patterns. Virology, 272(1): 127–136.
[6]Grant, A., Seregin, A., Huang, C., Kolokoltsova, O., Brasier, A., Peters, C., & Paessler, S. 2012. Junín Virus Pathogenesis and Virus Replication. Viruses, 4 (10): 2317–2339.
[7]Martinez, M. G., Cordo, S. M., & Candurra, N. A. 2007. Characterization of Junín arenavirus cell entry. Journal of General Virology, 88(6): 1776–1784.
[8]Hallam, S. J., Koma, T., Maruyama, J., & Paessler, S. (2018). Review of Mammarenavirus Biology and Replication. Frontiers in Microbiology, 9: 1751.
[9]Castilla, V., Mersich, S. E., Candurra, N. A., & Damonte, E. B. 1994. The entry of Junin virus into Vero cells. Archives of Virology 136 (3–4): 363–374.
[10]Gómez, R. M., Giusti, C. J. de, Vallduvi, M. M. S., Frik, J., Ferrer, M. F., & Schattner, M. 2011. Junín virus. A XXI century update. Microbes and Infection, 13(4): 303–311.
[11]Candurra, N. A., & Damonte, E. B. 1997. Effect of inhibitors of the intracellular exocytic pathway on glycoprotein processing and maturation of Junin virus. Archives of Virology 142 (11): 2179–2193.
[12]Ziegler, C. M., Eisenhauer, P., Kelly, J. A., Dang, L. N., Beganovic, V., Bruce, E. A., King, B. R., Shirley, D. J., Weir, M. E., Ballif, B. A., & Botten, J. 2018. A Proteomics Survey of Junín Virus Interactions with Human Proteins Reveals Host Factors Required for Arenavirus Replication. Journal of Virology, 92(4): e01565-17.
[13]Zong, M., Fofana, I., Choe, H., & Perlman, S. 2014. Human and Host Species Transferrin Receptor 1 Use by North American Arenaviruses. Journal of Virology, 88(16): 9418–9428.
[14]Hickerson, B. T., Sefing, E. J., Bailey, K. W., Van Wettere, A. J., Penichet, M. L., & Gowen, B. B. 2020. Type I interferon underlies severe disease associated with Junín virus infection in mice. ELife: 9: e55352.
[15]Huang, C., Walker, A. G., Grant, A. M., Kolokoltsova, O. A., Yun, N. E., Seregin, A. V., & Paessler, S. 2014. Potent inhibition of Junín virus infection by interferon in murine cells. PLoS Neglected Tropical Diseases, 8(6): e2933–e2933.
[16]Seregin, A. V., Yun, N. E., Miller, M., Aronson, J., Smith, J. K., Walker, A. G., Smith, J. N., Huang, C., Manning, J. T., de la Torre, J. C., & Paessler, S. 2015. The glycoprotein precursor gene of Junin virus determines the virulence of the Romero strain and the attenuation of the Candid #1 strain in a representative animal model of Argentine hemorrhagic fever. Journal of Virology, 89(11): 5949–5956.
[17]Scolaro, L. A., Mersich, S. E., & Damonte, E. B. 1990. A mouse attenuated a mutant of junin virus with an altered envelope glycoprotein. Archives of Virology, 111(3-4): 257–262.
[18]Lander, H. M., Grant, A. M., Albrecht, T., Hill, T., & Peters, C. J. 2014. Endothelial Cell Permeability and Adherens Junction Disruption Induced by Junin Virus Infection. The American Journal of Tropical Medicine and Hygiene, 90(6): 993–1002.
[19]Gowen B. B., Hickerson B. T., York J., Westover J. B., Sefing E. J., Bailey K. W., Wandersee L., Nunberg J. H., & Heise M. T. 2021. Second-Generation Live-Attenuated Candid#1 Vaccine Virus Resists Reversion and Protects Against Lethal Junín Virus Infection in Guinea Pigs. Journal of Virology, 95(14): e0039721.
[20]Wikipedia contributors. (2021, June 7). Argentinian mammarenavirus. In Wikipedia, The Free Encyclopedia. Retrieved September 19, 2021, from https://en.wikipedia.org/w/index.php?title=Argentinian_mammarenavirus&oldid=1027284857
[21]Charrel RN, Lamballerie Xde (2003). Arenaviruses other than Lassa virus. Antiviral Research 57:89–100.
[22]Krauss, H., Weber, A., Appel, M., Enders, B., Isenberg, H. D., Schiefer, H. G., Slenczka, W., von Graevenitz, A., & Zahner, H. (2003). zoonoses: Infectious diseases transmissible from animals to humans, 3rd edition. Medical Microbiology and Immunology 194:219–220.
[23]Charrel RN, de Lamballerie X. Arenaviruses other than Lassa virus. Antiviral Res. 2003 Jan; 57(1-2):89-100.
