Klebsiella pneumoniae pathogenesis

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gammaproteobacteria
| Order = Enterobacteriales
| Family = Enterobacteriaceae
| Genus = Klebsiella
| Species = K. pneumoniae [1]
|
NCBI: Taxonomy |
|
NCBI: Complete genome here |
Description
Klebsiella pneumoniae is a gram-negative, non-motile, lactose fermenting, rod-shape organism. K. pneumoniae is able to grow either with or without free oxygen, deeming it a facultative anerobe. This organism is also surrounded by a capsule, which increases its virulence by acting as a physical barrier to evade the host’s immune response. This capsule also protects the cell from dessication. K. pneumoniae is a home-grown microorganism in that it is resides in the microbiota of humans. It can be found in the mouth, skin, and intestinal tract, where it initially does not cause disease. Although found in the microbiota, K. pneumoniae can progress into severe bacterial infections leading to pneumonia, bloodstream infections, wound infections, urinary tract infections, and meningitis. Patients who require equipment such as catheters or ventilators are at high risk for infections. Also, a patient administered a course of broad-spectrum antibiotic treatment is at an even high risk due to the disruption of the normal flora of the bacteria in the body, deeming it more susceptible to pathogens [1].
Klebsiella pneumoniae was named afer Edwin Klebs (1834-1913), a 19th century German microbiologist [2]. Although he was not the first to isolate K. pneumoniae, naming a genus after him honored his work with Corynebacterium diptheriae. Around the same time, Hans Christian Gram (1853-1938), created a microbiological technique known as the Gram stain in 1884 to differentiate between K. pneumoniae and S. pneumoniae [3].
Pathogenesis
Transmission
Klebsiella infections is spread through exposure to the bacteria via respiratory tract, which causes pneumonia, or the blood to cause an infection in the bloostream. Klebsiella infections are most well-known in hospitals spread through person-to-person contact by contaminated hands of surrounded people in the hospitals, whether it be an employee or a patient. Klebsiella is spread very easily and rapidly, but not through the air. Healthcare settings are most vulnerable to Klebsiella infections due to the nature of procedures that allow easy access of bacteria into the body. Patients who are on ventilators, catheters, or surgery wounds are highly prone to catching this deadly infection [4].
Incubation/Infectious Dose/Colonization
In humans, the infectious dose is not known. As for the incubation period, it is also not fully understood but possibly arises within a number of days [4]. Klebsiella bacteria are found widely through nature in soil and water. In regards to human, K. pneumoniae is prevalent in normal microbiota of the intestinal tract and colon, but not in alarmingly high numbers. They may also be found in the mouth and the skin [3]. Infection of K. pneumonia occur in the lungs, where they cause necrosis, inflammation, and hemorrhage within the lung tissue. This is caused by aspirating oropharyngeal microorganisms into the lower respiratory tract. Hospital-acquired infections rely on the urinary tract, lower respirator tract, biliary tract, and surgical wounds to set up colonization [5].
Epidemiology
When dealing with hospital-acquired bacterial infections caused by K. pneumoniae can arise in different parts of the body and in different forms of illness depending on transmission. K. pneumoniae is responsible for 6-17% of UTI’s, 7-14% of pneumonia, 4-15% of septicemia, 2-4% of wound infections, 4-17 nosocomial infections in intensive care units, and 3-20% of all neonatal septicemia cases. All of these cases rank within at least the top 11 in comparison to all other bacterial pathogens. In the United States, people who suffer from alcoholism make up 66% of people affected by community-acquired pneumonia. K. pneumoniae is now among the top 8 pathogens in hospitals and is a rising issue among hospitals all around the world due to antibiotc resistance. In humans, K. pneumoniae resides in the nasopharynx and in the intestinal tract. Since gram-negative bacteria do not have good growth on human skin, they are rarely found there in comparison to internal parts of the body. Reported carrier rates are quire the opposite of this fact, when in a hospital environment. Carriers rated in hospitalized patients were 19% in the pharynx, 77% in the stool, and 42% on the hands. Even hospital employees had elevated rates of carriage to K. pneumoniae. These findings were linked to the over usage of broad-spectrum antibiotics rather than delivery of care [6]. In 2011, an investivation of Klebsiella pneumonia carbapenemase (KPC)-producing Enterobacteriaceae was conducted in hospitals among patient with short and long-term stays. Over a 1-year period, KPC-producing Enterobacteriaceae was found throughout 4 counties in Indiana and Illinois. The source of the problem was found to be within long-term facilities and patients [5]. KPC has been found in a total of 44 states thus far [7].
14% of bacteremia cases are because by K. pneumoniae, which places it in second place next to Escherichia coli for origins of gram-negative sepsis. Outbreaks of neonatal septicemia and K. pneumoniae can be found worldwide. In Israel, a number of hospital facilities reported increases in KPC-producing Enterobacteriaceae beginning in 2006, while the first case in the United States was reported in 2001[8].
Virulence Factors
Capsular Polysaccharide and Lipopolysaccharide O Side Chain
Lipopolysaccharide (LPS) and capsular polysaccharide (CPS) are two of the most important virulence factor of K. pneumoniae in causing sepsis. To resist complement-mediated killing, LPS contains lipid A, core, and O-polysaccharide antigen. CPS is essentially the outer layer of the pathogen containing polymorphonuclear cells, which creates resistance against phagocytosis. CPS reduced interaction between bacterial cells by reducing the amount of C3 being put on the bacteria and by acting as a barrier to obstruct contact between macrophage receptors and their ligands on the bacterial surface. CPS is absolutely critical and also modulates interaction between surfactanct protein D (SP-D) and K. pneumoniae. SP-D mediates aggregation and improves phaocytic clearance by human alveolar macorphages. With CPS, C3 and SP-D cannot attach, which creates clearant of the microorganism from the lower respiratory tract, resulting in pneumonia. (http://iai.asm.org/content/70/5/2583.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=Molecular+analysis+of+the+contribution+of+the+capsular+polysaccharide+and+the+li&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT)
Capsular Antigens
K. pneumoniae use capsular antigens composed of complex acidic polysacchardes, which are essential to the virulence to this pathogen. The capsular antigens can be classified into 77 serological types and are often made up of uronic acids. As a mechanism of protection, the bacterium evades phagocytosis by utilizing thick bundles of fibrillous structures by polymorphonuclear granulocytes. This mechanism also prevents killing by bactericidal serum factors and inhibits complement constituents, such as C3b, which opsonize the pathogen. These effective virulence factors also have the ability to inhibit the differentiation capacity of macrophages in vitro.
Endotoxin
First, the presence of cell wall receptors enables K. pneumoniae to attach to the host cell, thereby altering the bacterial surface so that phagocytosis by polymorphonuclear leukocytes and macrophages is impaired and invasion of the non-phagocytic host cell is facilitated. Second, invasion of the host cell is also facilitated by the large polysaccharide capsule surrounding the bacterial cell; in addition this capsule acts as a barrier and protects the bacteria from phagocytosis. Third, K. pneumoniae produces an endotoxin that appears to be independent of factors that determine receptors and capsular characteristics. Marked interspecies differences in endotoxin production may correlate with virulence. Although some or all of these factors may ultimately determine virulence, the interaction of these factors in vivo has made it difficult to assess the relative contribution of any one of these virulence factors. The pathogenic mechanisms of K. pneumoniae that ultimately determine virulence remain unclear and will require further study. (SITE THIS!!) K Antigen?
Antibiotic Resistant Strains
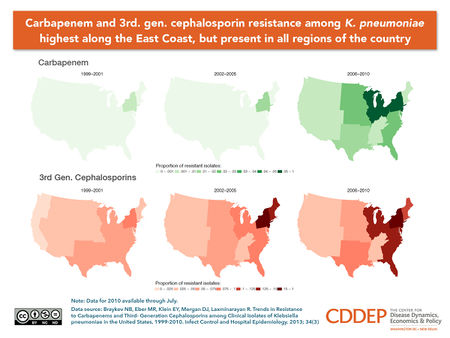
KPC Outbreak at NIH
In 2011, Klebsiella pnuemomiae Carbapenamase arrived in one of the most established and renowned hospitals, the Clinical Center at the National Institutes of Health in Bethesda, Maryland, known as the NIH. A 43-year-old woman was transferred to the NIH, who had lung transplant complications and a KPC infection. With no known, outbreak, NIH employee were relieved after clearing the infection and discharging the patient. Weeks later, a new patients tested positive for KPC, but with no know link to patient number one. Over the next six months, several patients were showing up positive for KPC and eventually quarantined from the rest of the hospital. Doctors began administering combination antibiotics, older formulas from decades back, and even experimental antibiotics. None of these methods seemed to work and ultimately, a total of 18 patients became infected with KPC. 6 patients died from KPC and the outbreak came to a halt just as fast as it started. Genetic analysis was used to understand the transmission sequence, The bacteria is thought to be home grown in that we have K. pneumoniae living in our digestive systems with a gene that can spread resistance to different bacteria. Today, the NIH knows that KPC is still lurking in the hospital. They only know that they must be extremely vigilant in the future due to the increasing rise of KPC infections in the United States. (PBS)
Clinical Features
Symptoms
Klebsiella infections are prominent in individuals who have diabetes or who suffer from alcoholisms due to a weakened immune system. K. pneumonia is also a well-known cause of community-acquired pneumonia. Symptoms associated with community-acquired K. pneumoniae include sudden onset, high fever and a jelly-like sputum. Shortness of breath and coughing are also common symptoms.I f introduced into the bloodstream, K. pneumoniae has the capability of causing meningitis, which affect the CNS. Symptoms include sharp head pain, nausea, dizziness, and impaired memory. Urinary tract infections alongside bacteremia are leading consequences of Klebsiella infections as well. An alarming issue and even more common infection originating from K. pneumoniae are nosocomial infections.
Hospital patients who have extended stays in Intensive Care Units, who are either receiving long courses of broad spectrum antibiotics or have additional health issues are at risk for attaining a nocosomial infection. Broad spectrum antibiotics introduced into the microbiome, in turn, creates antibiotic resistance for K. pneumoniae to thrive and colonize. Many hospital patients and even faculty members who acquire K. pneumoniae infections will develop pneumonia, bacteremias, and urinary tract infections. (lousiana). The emergence of CRE producing the Klebsiella pneumonia carbapenase (KPC) enzyme are not increasingly common all around the United States. Antibiotic resistance is now a serious problem in hospitals, withincreased mortality rates and decreased options of treating the infection. (IMP producing)
Diagnosis
K. pneumonia may be isolated from blood, urine, pleural fluid, and wounds. By simply gram staining a sputum sample obtained from a patient could lead one to diagnosing K. pneumoniae. Cultures should be obtained from sites such as open wounds, peripheral or central intravenous access sites, urinary catheters, and respiratory equipment. Chest radiography can also lead the way in diagnosing a K. pneumoniae infection. The organism usually resides in one of the upper lobes of the lungs, but may also be involved in lower portions as well. The lobe will appear swollen and can, in many cases, produce abscesses.
Treatment
Antibiotic Therapy
Unfortunately, K. pneumoniae is resistant to a number of antibiotics, deeming treatment options very limited. Choosing an antibiotic treatment for K. pneumoniae depends on the organ system that has been targeted. The choice is especially modified for people with confirmed bacteremia. Antibiotics with high intrinsic activity against K. pneumoniae include cephalosporin, carbapenems, aminoglycosides, and quinolones. These treatments are initially used as monotherapy or even as a combination. For patients who are severely ill, an initial course, usually between 48-72 hours of combination aminoglycoside therapy, is suggested. This should then be followed by an extended-spectrum cephalosporin. (http://emedicine.medscape.com/article/219907-treatment#a1127)
Resistance
Carbapenem resistance is a emerging issue and is notably due to K. pneumoniae carbapenemase beta-lactamase (KPC). With the spread of KPC-producing bacteria, clinicians are dependent on polymyxins and tigecycline for treatment. Polymyxins have been the only agents active against KPC-producing bacteria. However, they were used infrequently due to their association with nephrotoxicity and neurotoxicity. Only a small amount of data support Polymyxin as a quality treatment for KPC. During a Manhattam outbreak caused by KPC-producing K. peumoniae, three bloodstream infections were treated with one survivor from the group. This drug is often used in combination with other antimicrobials. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075864/)
Surgery
Surgey may be needed for patients who experience empyema, lung anscess, pulmonary gangrene, or respiratory tract obstruction following a Klebsiella infection. Correction of posterior urethral valves in patients with reoccurring UTIs is a possibility or other abnormalities influenced by infection. (http://emedicine.medscape.com/article/219907-treatment#a1128)
Prevention
To prevent the spread of infections, patients should remain very cautious of their handwashing habits. Handwashing with soap and water should happen in the following instances: before touching eyes, nose, or mouth, before preparing food, before addressing bandaged or wound areas, after using the restroom, and after using the restroom. It is especially important to be cautious when entering and exiting hospital rooms. Be sure to use proper handwashing techniques after touching doorknobs, bed rails, using hospital restroom, and interacting with sick patients, even if they are loved ones.
Host Immune Response
IL-12/23 p40, interferon γ (IFN-γ), and IL-17 are critical for host defense against Klebsiella pneumoniae.
References
1. UniProt. Taxonomy: Species Klebsiella pneumoniae. Available at http://www.uniprot.org/taxonomy/990925.
2. Centers for Disease Control and Prevention. Klebsiella pneuminae in Healthcare Settings. Available at http://www.cdc.gov/HAI/organisms/klebsiella/klebsiella.htm.
3. Department of Health and Hospitals. Available at http://new.dhh.louisiana.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/EpiManual/KlebsiellaManual.pdf.
4. Boston Medical Research Occupational Health Program. Available at http://www.bu.edu/rohp/files/2012/08/KPC-Klebsiella.pdf.
5. Medscape. Klebsiella Infections. Available at http://emedicine.medscape.com/article/219907-overview#a01995.
6. Klebsiella spp. As Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors Clin Microbial Rev. Oct 1998; 11(4): 589-603.
7. A Superbug Outbreak At NIH. Available at http://www.pbs.org/wgbh/pages/frontline/health-science-technology/hunting-the-nightmare-bacteria/a-superbug-outbreak-at-nih/.
8. Carbapenem-Resistant Enterobacteriaceae: Epidemiology and Prevention. Oxford Jounals. Clinical Infectious Diseases. Mar 2011; 53(1): 60-67.
9. Hunting the Nightmare Bacteria. Available at http://www.pbs.org/wgbh/pages/frontline/hunting-the-nightmare-bacteria/.
10. American Society for Microbiology: Infection and Immunity. Molecular Analysis of the Contribution of the Capsular Polysaccharide and the Lipopolysaccharide O Side Chain to the Virulence of Klebsiella pneumoniae in a Murine Model of Pneumonia. Available at http://iai.asm.org/content/70/5/2583.full?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&titleabstract=Molecular+analysis+of+the+contribution+of+the+capsular+polysaccharide+and+the+li&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT.
Created by Victoria Ann Kappel
Student of Dr. Tyrrell Conway, University of Oklahoma
