Kyasanur Forest Disease Virus: Difference between revisions
| Line 12: | Line 12: | ||
=Morphology= | =Morphology= | ||
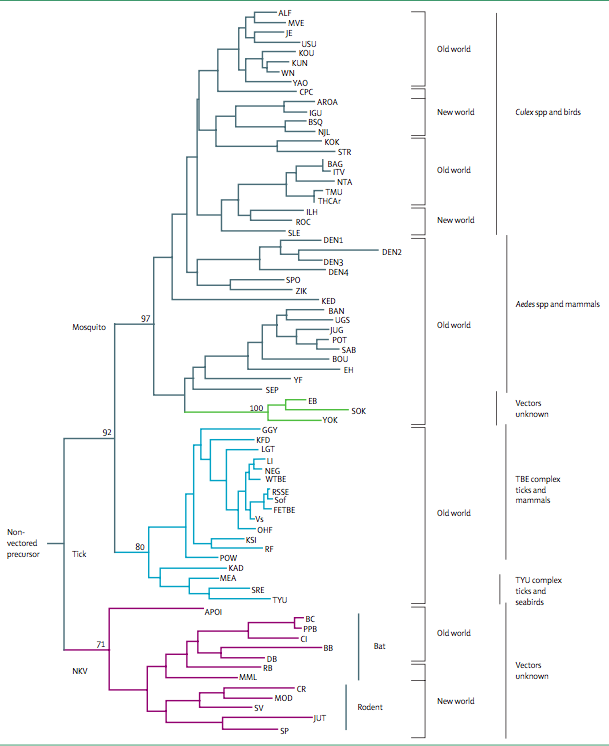
KFDV is pathogenic member of tick-borne encephalitis virus group (TBEV). This group consists of related viruses such as the Louping ill, Omsk hemorrhagic fever, Langat, Royal Farm, Gadgets Gully, and Powassan viruses (Mehla, 2009). There is a wide range of tick species contributing to the tick-borne KFDV group, mainly the Haemaphysalis spinigera. Mature TBEVs have a diameter of 50 nm and contain 3 structural proteins for the capsid, envelope and membrane (Heinz & Stiasny, 2012). These viruses enter the cell via receptor-mediated endocytosis and undergo an immature and mature phase inside the cell (Heinz & Stiasny, 2012). A recent study showed that infection of KFDV is lethal in mice and leads to elevated levels of pro-inflammatory cytokines in the brain. However, not virus was detected in visceral organs (Sawatsky et. al., 2014). | KFDV is pathogenic member of tick-borne encephalitis virus group (TBEV). This group consists of related viruses such as the Louping ill, Omsk hemorrhagic fever, Langat, Royal Farm, Gadgets Gully, and Powassan viruses (Mehla et. al., 2009). There is a wide range of tick species contributing to the tick-borne KFDV group, mainly the Haemaphysalis spinigera. Mature TBEVs have a diameter of 50 nm and contain 3 structural proteins for the capsid, envelope and membrane (Heinz & Stiasny, 2012). These viruses enter the cell via receptor-mediated endocytosis and undergo an immature and mature phase inside the cell (Heinz & Stiasny, 2012). A recent study showed that infection of KFDV is lethal in mice and leads to elevated levels of pro-inflammatory cytokines in the brain. However, not virus was detected in visceral organs (Sawatsky et. al., 2014). | ||
=Ecology= | =Ecology= | ||
Revision as of 15:39, 2 December 2015
Classification
Flaviviridae; flavivirus
Description and significance
The Kyasanur Forest Disease (KFD) is a tick-borne viral hemorrhagic fever (Murhekar et. al., 2015). First discovered in 1956, is endemic to forested areas of southern India. Originally indigenous to the Shimoga district in the Karnataka state, it has gradually spread to other parts of southern Asia causing epidemics in the recent decades (Mourya et. al., 2012; Nichter 1987). A major driving factor in its eminence was deforestation which brought monkeys in closer contact to humans (Gould et. al,. 2008). The KFD virus (KFDV) was first identified in sick monkeys of the Kyasanur forest during a series of epizootic outbreaks. The black-faced langur (Presbytis entellus), the red-faced bonnet monkey (Macaca radiata) and humans are susceptible to KFDV. Ticks act as a vector for the virus. They feed on the blood of the infected organism and fall off when the host dies, creating a hotspot for infection of humans (Mehla et. al., 2009). Persons with occupational exposures to the forest (i.e., herders, farmers, forest workers, hunters) are especially at a high risk for infection. Consequently, KFD has become a concern for public health authorities of the area and surrounding locations (Mehla et. al., 2009).
Recently, research of KFDV has focused on transmission, diagnostic procedures and vaccination. Efforts are continually made to determine patterns of transmission in order to rule out human to human contagion possibility. Fortunately, nosocomial infections have never been reported (Mehla et. al., 2009). The improvement of accurate and promptly accessible diagnostic tests have given health officials greater ability to treat the infection and the development of vaccines and vaccination strategies allow for additional prevention (Mourya et. al., 2012; Kasabi et. al., 2013). However, the current vaccine is not very effective (Murhekar et al., 2015). Furthermore, the virus has been tracked to parts of China (Wang, 2009) and closely related species to Saudi Arabia (Mehla et. al., 2009), indicating the necessity to improve awareness and knowledge of KFD. Despite the efforts made to control and track KFDV, the virus still causes large numbers of cases of encephalitis and hemorrhagic fever across patients (Pattinaik, 2006). With increasing global temperatures and the transport of goods across the world, it is imperative to regulate its spread and the spread of other flaviviruses (Gould et. al., 2008).
Genome structure
As part of the family Flaviviridae, this virus contains a spherical positive single-stranded RNA molecule of 11,000 bases in length (Chambers et al. 1990; Pattnaik, 2006; Heinz & Stiasny, 2012).
Morphology
KFDV is pathogenic member of tick-borne encephalitis virus group (TBEV). This group consists of related viruses such as the Louping ill, Omsk hemorrhagic fever, Langat, Royal Farm, Gadgets Gully, and Powassan viruses (Mehla et. al., 2009). There is a wide range of tick species contributing to the tick-borne KFDV group, mainly the Haemaphysalis spinigera. Mature TBEVs have a diameter of 50 nm and contain 3 structural proteins for the capsid, envelope and membrane (Heinz & Stiasny, 2012). These viruses enter the cell via receptor-mediated endocytosis and undergo an immature and mature phase inside the cell (Heinz & Stiasny, 2012). A recent study showed that infection of KFDV is lethal in mice and leads to elevated levels of pro-inflammatory cytokines in the brain. However, not virus was detected in visceral organs (Sawatsky et. al., 2014).
Ecology
Habitat; symbiosis; contributions to the environment.
Transmission
Pathology and Diagnostics
How does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
Vaccines and Prevention
Current Research in Related Viruses
Include information about how this microbe (or related microbes) are currently being studied and for what purpose
References
Banerjee K. Kyasanur Forest disease. The Arbo-Viruses: Ecology and Epidemiology (Vol. 3), Monath TP (ed.). CRC Press: Boca Roton, FL, 1988; 93–116.
Boshel-M J. Kyasanur forest disease: ecological considerations. Am J Trop Med Hyg 1969; 18: 67–80.
Chambers TJ, Hahn CS, Galler R. Rice CM. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44:649–688.
Domingo, E., Escarmis, C., Sevilla, N. & Martinez, M.-A. (1997) Population dynamics and molecular evolution of RNA viruses. Factors in the Emergence of Arbovirus Diseases, pp. 273–278. Edited by J. F. Saluzzo & B. Dodet. Paris: Elsevier.
Gould EA, Solomon T. Pathogenic flaviviruses. Lancet 2008; 371: 500–509.
Heinz, Franz X., and Karin Stiasny. Flaviviruses and Flavivirus Vaccines. Vaccine 30.29 (2012): 4301-306. Web.
Kasabi, Gudadappa S., et al., Coverage and Effectiveness of Kyasanur Forest Disease (KFD) Vaccine in Karnataka, South India, 2005–10. PLoS Negl Trop Dis PLoS Neglected Tropical Diseases 7.1 (2013): n. pag. Web.
Kiran, S.K., et al., Kyasanur Forest disease outbreak and vaccination strategy, Shimoga District, India, 2013-2014. Emerg Infect Dis, 2015. 21(1): p. 146-9.
"Kyasanur Forest Disease (KFD)." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 23 Dec. 2013. Web. 20 Oct. 2015.
Mehla, R., et al., Recent Ancestry Of Kyasanur Forest Disease Virus. Emerging Infectious Diseases, 2009. 15(9): p. 1431-1437.
Mourya, D.T., et al., Diagnosis of Kyasanur forest disease by nested RT-PCR, real-time RT-PCR and IgM capture ELISA. J Virol Methods, 2012. 186(1-2): p. 49-54.
Murhekar, M.V., et al., On the transmission pattern of Kyasanur Forest disease (KFD) in India. Infect Dis Poverty, 2015. 4: 37.
Nichter, Mark Kyasanur Forest Disease: An Ethnography of a Disease of Development. Medical Anthropology Quarterly. New Series, Vol. 1, No. 4 (Dec., 1987), pp. 406-423
Pattnaik, P. (2006). Kyasanur forest disease: An epidemiological view in India. Reviews in Medical Virology, 2006. 16 (3), 151-165.
Sawatsky, Bevan, Alexander J. Mcauley, Michael R. Holbrook, and Dennis A. Bente. Comparative Pathogenesis of Alkhumra Hemorrhagic Fever and Kyasanur Forest Disease Viruses in a Mouse Model. PLoS Negl Trop Dis PLoS Neglected Tropical Diseases. 2014 Jun; 8(6): e2934
Wang J, Zhang H, Fu S, Wang H, Ni D, Nasci R, et al. Isolation of Kyasanur forest disease virus from febrile patient, Yunnan, China. Emerg Infect Dis. 2009. 15(2): 326–8. doi:10.3201/eid1502.080979.