Lentiviral Vectors in Gene Therapy: Difference between revisions
No edit summary |
No edit summary |
||
| Line 19: | Line 19: | ||
Retroviruses are characterized by their use of the host cell's reverse transcriptase to create DNA from viral RNA. The DNA is then incorporated into the host's DNA genome. Viruses in the genus <i>Lentivirus</i> have slow incubation periods and are categorized into five different serotypes based on their host: primates, sheep/goats, horses, cats, and cattle. RELIK was found to be the first endogenous lentivirus, infecting rabbits, dating millions of years [x]. Present lentiviruses are exogenous, incorporating their DNA into a host's upon infection, and they can infect non-dividing cells, unlike most other retroviruses. | Retroviruses are characterized by their use of the host cell's reverse transcriptase to create DNA from viral RNA. The DNA is then incorporated into the host's DNA genome. Viruses in the genus <i>Lentivirus</i> have slow incubation periods and are categorized into five different serotypes based on their host: primates, sheep/goats, horses, cats, and cattle. RELIK was found to be the first endogenous lentivirus, infecting rabbits, dating millions of years [x]. Present lentiviruses are exogenous, incorporating their DNA into a host's upon infection, and they can infect non-dividing cells, unlike most other retroviruses. | ||
<br> | <br> | ||
After a long incubation period and subsequent cell divisions and virus gene proliferation, lentiviruses cause prolonged illness, such as AIDS in humans. Much is known about the method of infection of lentiviruses. | After a long incubation period and subsequent cell divisions and virus gene proliferation, lentiviruses cause prolonged illness, such as AIDS in humans. Much is known about the method of infection of lentiviruses. Glycoproteins (gp) on the envelope of the virus recognize antibody receptors on host cells and attach to them, prompting membrane fusion of the two. Viral RNA is released from the capsid in the cytoplasm of the cell and host cell reverse transcriptase beings to synthesize complementary DNA. The double-stranded DNA is taken into the nucleus and integrated into the host's genome. It can lay dormant until necessary transcription factors are present to begin transcription of the viral DNA. As long as the viral DNA lays dormant within the host's genome, the host will duplicate it during cell division and all lineages thereafter will contain the viral DNA. When the viral DNA undergoes transcription, it will leave the nucleus and the cell's mechanisms will translate the RNA into viral proteins, eventually assembling new virions that erupt from the cell, killing it. | ||
[[Image:RNA.jpg|thumb|300px|left| The retrovirus infection cycle. [http://www.nature.com/mt/journal/v13/n6/fig_tab/mt2006127f2.html].]] | [[Image:RNA.jpg|thumb|300px|left| The retrovirus infection cycle. [http://www.nature.com/mt/journal/v13/n6/fig_tab/mt2006127f2.html].]] | ||
<br> | <br> | ||
Revision as of 04:15, 16 November 2012
A Viral Biorealm page on the family Lentiviral Vectors in Gene Therapy
Lentiviruses are some of the most recognizable–and life threatening–vertebrate viruses. Viruses in this genus include human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), and feline immunodeficiency virus (FIV). Their exogenous characteristics make them incredibly ideal for use in gene therapy.
Gene therapy requires delivery vehicles to transfer a gene into an organism's chromosome. Lentiviral vectors are highly successful in permanently changing the target cell and thus increase the efficiency of therapeutic treatment. Hemophilia and other blood-related diseases, AIDS, and various cancers are current targets of lentiviral vectors and they can be used in conjunction with other antiviral and anticancer drugs.
Gene Therapy
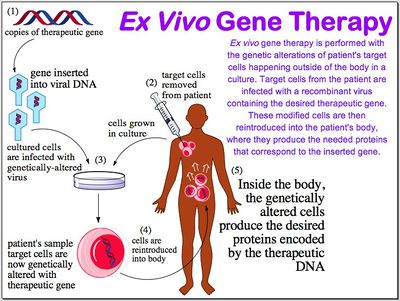
Genetic disorders result from mutated or missing genes. The goal of gene therapy is to repair the flawed DNA by completely repairing it-in most cases the expectation is a total replacement of the gene. Reparation at the source of the disorder impacts subsequent cells and tissues down the line instead of simply treating the symptoms of the disorder. In order to repair the disorder at its source, there must be an efficient way to gain entry into the cells and permanently incorporate the new gene into the target cell's genome. Various methods exist for this delivery and success depends on the nature of the disorder. What genes are involved, how many genes are involved, how the patient's immune system will react, and the physical effects of the mutated or missing gene has on a person must all be taken into account when considering gene therapy as a treatment option. If a disorder can be rectified by replacing the mutated gene, and the target cells are fairly accessible, then a suitable method of gene delivery will be devised.
"Vectors" refer to the method of gene insertion. Non-viral vectors are plasmids cultivated in bacteria and naked plasmids that are inserted directly into the cell. Non-viral vectors are not cell specific but are generally less effective in their integration into the host cell [x]. Viral vectors refer to modified viruses used to deliver DNA into target cells. Viral vectors are usually more specific to certain cell types but are limited in the size of the genome they can fit inside their capsid. Viral vectors may cause an immune response in the patient, or if the patient is inherently immune to the specific type of virus, then the success of DNA integration is diminished.
There are also various ways in which target cells can be isolated for integration.Ex vivo requires a removal of target cells from the patient, growing them in a culture, and introducing the vector into the culture. After the target cells have integrated the DNA, they are replaced into the patient. In vivo treatment simply requires the injection of the vector that is specific to target cells into the patient. Since viral vectors are commonly regarded as the most effective delivery systems, many methods have been researched and utilized various types of viruses: retroviruses, adenoviruses, adeno-associated viruses, and the herpes simplex virus are common. Viruses can also be modified to express fewer capsid proteins and to express certain proteins that are specific to target cells.
Choosing the type of virus vector depends on the nature of the genetic reparation needed. For example, retroviruses only infect dividing cells but subsequent cell lineages will contain the inserted DNA. DNA carried by adenoviruses and the herpes simplex virus will not incorporate into the host cell's DNA and may be discarded or degraded by the host cell after a period of time. For this reason, focus has shifted into retroviral vectors, specifically lentiviruses.
About Lentiviruses
Retroviruses are characterized by their use of the host cell's reverse transcriptase to create DNA from viral RNA. The DNA is then incorporated into the host's DNA genome. Viruses in the genus Lentivirus have slow incubation periods and are categorized into five different serotypes based on their host: primates, sheep/goats, horses, cats, and cattle. RELIK was found to be the first endogenous lentivirus, infecting rabbits, dating millions of years [x]. Present lentiviruses are exogenous, incorporating their DNA into a host's upon infection, and they can infect non-dividing cells, unlike most other retroviruses.
After a long incubation period and subsequent cell divisions and virus gene proliferation, lentiviruses cause prolonged illness, such as AIDS in humans. Much is known about the method of infection of lentiviruses. Glycoproteins (gp) on the envelope of the virus recognize antibody receptors on host cells and attach to them, prompting membrane fusion of the two. Viral RNA is released from the capsid in the cytoplasm of the cell and host cell reverse transcriptase beings to synthesize complementary DNA. The double-stranded DNA is taken into the nucleus and integrated into the host's genome. It can lay dormant until necessary transcription factors are present to begin transcription of the viral DNA. As long as the viral DNA lays dormant within the host's genome, the host will duplicate it during cell division and all lineages thereafter will contain the viral DNA. When the viral DNA undergoes transcription, it will leave the nucleus and the cell's mechanisms will translate the RNA into viral proteins, eventually assembling new virions that erupt from the cell, killing it.
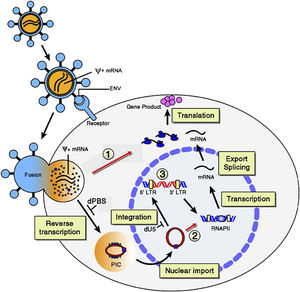
Lentiviral Vectors
Successful gene therapy requires permanent expression of the gene of interest in the target cell. The efficiency of the mode of gene delivery obviously correlates to the success of DNA integration into the host. Lentiviruses have been of interest for many years [x] because of their notoriously successful infection rate. The long terminal repeat (LTR) region of HIV-1 is a target area for modification; the sequence is a mediator for virus DNA integration into host cells. Env proteins can be modified with ligands and antibodies to target a more specific cell type [x]. In vivo cell targeting is certainly the most complicated of the delivery methods but can produce successful noninvasive results more quickly if honed.
One method involves specific antibodies on the envelope of the virus recognizing target cell surface molecules (such as αCD20 on the viral envelope recognizing CD20 on the target cell; CD20 is an antibody on a B-cell), and a separate, modified, fusion glycoprotein attaches to the host cell [x]. **FIGURE 5 FROM YANG** The virus is taken into the cell via endocytoses. The virus is within an endosome and fuses to its membrane, prompting the viral and endosome membranes to open and the capsid is released into the cell.
Current Lentiviral Vectors
HIV X-Linked Adrenoleukodystrophy
Potential Side Effects/Safety
Using foreign vectors in a patient always runs the risk of a severe adverse immune reaction. The "randomness" of the insertion of the lentiviral vector's DNA can potentially disrupt existing normal genes. Virus proteins on its capsid can be removed, leaving only a few and reducing the immune response of the patient.
Future Directions of Lentiviral Vectors
Various pharmaceutical and research companies focus solely on lentiviral gene therapy
see http://www.lentigen.com/
stem cells
vaccines
T cell therapies
proteins?
References
[1] "Tools of the Trade." Learn Genetics. Genetic Science Center, University of Utah, n.d. Web. Nov. 2012. <http://learn.genetics.utah.edu/content/tech/genetherapy/gttools/>. [2] Katzourakis, A., M. Tristem, O. G. Pybus, R. J. Gifford. 2007. "Discovery and analysis of the first endogenous lentivirus." Proceedings of the NAtional Academy of Sciences of the United States of America. 104: 6261-6265. [3] Somia N. V. , Zoppe M. , Verma I. M. 1995. "Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery." Proc. Natl. Acad. Sci. USA 92:7570–7574.
[4] Naldini, L. 1998. "Lentiviruses as gene transfer agents for delivery to non-dividing cells." Current Opinion in Biotechnology. 9: 457-463.
[5] Yang, L., L. Bailey, D. Baltimore, P. Wang. 2006. "Targeting lentiviral vectors to specific cell types in vivo." Proc. Natl. Acad. Sci. USA 103:11479-11484.
Page authored by Irene McIntosh for BIOL 375 Virology, December 2012
