Link Between Microbes and Obesity
Introduction
Introduce the topic of your paper. What microorganisms are of interest? Habitat? Applications for medicine and/or environment?
Gut Microbiota
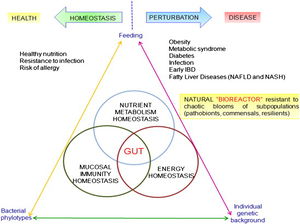
The role of the gut microbiome is the focus of renewed interest in health and disease. There has been an increasing number of scientific articles published on intestinal microbiota each year over the past several decades. Most studies were devoted to understanding the impact in the intestinal environment, however, new studies are looking at the effect of commensal microbes on the mammalian gut. The human gut microbiota is composed of microorganisms that live in the digestive tract and is considered the largest supply of the human flora. The composition of the human gut microbial community is host specific. The microbial community is continually evolving due to exogenous and endogenous modifications. The microbiota can be at the center of causing many diseases which can have serious effects on all organ systems in the body. The compositional make-up of the microorganisms in the human gut is an important determinant of maintaining homeostasis or not.
The human body serves as beneficial host for many microorganisms including: bacteria, archaea, viruses, and unicellular eukaryotes. The microbiota refers to the group of microorganisms living in peaceful coexistence within their host. The human microbiota holds as many as 10¹⁴ bacterial cells. The human gut, a very large organ, has the surface size of approximately 200 m². Therefore, the human gut is an important surface for microbial colonization, along with GIT which is rich in microbial nutrients.
The gut microbiota is mostly composed of strict anaerobes; 99% of the bacteria in the gut are anaerobes. Two bacterial phyla, Bacteroidetes and Firmicutes, are the most predominant in the human gut. The following bacterial phyla: proteobacteria, verrucomicrobia, actinobacteria, fusobacteria, and cyanobacteria also have a smaller presence in the gut microbiota. The human gut microbiota can contain approximately 500 to 1,000 species and the collective human gut microbiota is composed of over 35,000 bacterial species. The intestinal microbiota is not homogeneous; the number of bacterial cells increases as it moves through the GI tract. 10¹ to 10³ bacteria per gram are present in the stomach and duodenum. The number of bacteria per gram increases to 10¹² in the colon, which is the final location of the GI tract. Different bacterial groups thrive at each different site in the intestine. For example, Bacteroides are found only in feces whereas Clostridium, Lactobacillus, and Enterococcus were found only in the mucus layer of the small intestine. The presence of colonized microbes in the human gut occurs immediately at birth. During the first year of life, the gut microbiota varies from human to human. However, after one year of age, the gut microbiota stabilizes and colonizes.
There are many contributing factors to the composition of the gut microbiota. Gut microbial composition can depend on host genetics, mutations, and diet based on studies of obesity.
Human gut-microbe populations fall into three distinct categories. The three categories are named after the dominant genus: Bacteroides, Prevotella, and Ruminococcus. Bacteroides are known for being able to break down carbohydrates. Prebotella are known for degrading the slimy mucus in the gut and will therefore lead to increased gut pain. Ruminococcus is commonly associated with weight gain because it helps cells absorb sugar. This revelation can help identify the causes of obesity and inflammatory bowel disease. The identification of a person’s gut type makes it possible for a person to determine what they can eat to stay slim and how well they metabolize.
Obesity
Obesity is the leading preventable cause of death worldwide. Obesity is a medical condition that affects 35.9% of the American population 20 years and older. Excess body fat accumulates and leads to adverse effects on health which can lead to a reduced life expectancy and increase health problems. An person is considered obese when their BMI, body mass index, exceeds 30 kg/m². Body mass index is determined by dividing a person’s weight in kilograms by the person’s height squared. Obesity increases the likelihood of the following diseases: heart disease, type 2 diabetes, obstructive sleep apnea, and osteoarthritis. Obesity occurs when a person consumes more calories than he or she burns. Therefore, the most common cause is a person eating too much and exercising too little. Other factors that play a role in obesity are: age, gender, genetics, environmental factors, physical activity, psychological factors, illness, and medication. Treatment options for obesity include dieting, physical exercise, and gastric bypass surgery. It is important to understand the mechanisms behind obesity and look to the correlation between the gut microbiota and obesity. Obese individuals tend to have a makeup of pathogens in their intestine that is different from that of people who are of normal weight.
Conclusion
This new field has shown the link between obesity and microorganisms living in the gut. Many current studies have shown how certain bacteria are responsible for an increase in weight. These studies are revolutionary because they show the importance of genetic and biotic factors that might be responsible for changes in weight. This new field can lead to new treatments on how to alter the gut microbiota to manage changes in weight.
Current Research
Human gut-microbe populations fall into three distinct categories. The three categories are named after the dominant genus: Bacteroides, Prevotella, and Ruminococcus. Bacteroides are known for being able to break down carbohydrates. Prebotella are known for degrading the slimy mucus in the gut and will therefore lead to increased gut pain. Ruminococcus help cells to absorb sugars which can be associated with weight gain. This revelation can help identify the causes of obesity and inflammatory bowel disease. The identification of a person’s gut type makes it possible for a person to determine what they can eat to stay slim and how well they metabolize.
Current research led by Andrew Gewirtz at Emory University indicate that the gut microbiota plays an important role in how your body stores the food you consume. They designed an experiment using lab mice to look at the relation between weight and gut bugs. Lab mice lacking protein, toll-like receptor 5, had a much higher percentage of the gut bugs in their system and were about 15% heavier. These mice had higher levels of inflammation, which was one of the main explanations for the extra weight. Lab mice were bred to lack a protein known as TLR₅, toll-like receptor 5. This protein sprouts on the surface of most intestinal cells. TLR₅ recognizes and binds to the whiplike flagella that bacteria use to move around; therefore, it controls the mass of pathogens living in the intestine. This protein sprouts on the surface of most intestinal cells. Without this protein, the normally harmless bacteria are able to multipy and expand in number. An increase in the number of these bacteria leads to an inflammatory state; the body tries to respond this increased number of bacteria while also making cells less sensitive to insulin. Intestinal cells need to balance between responding to inflammatory factors and insulin. Therefore, if a cell is busy responding to inflammatory factors triggered by the increase in bacterial numbers, these intestinal cells are more likely to forget to take up glucose. This desensitization to insulin and glucose results in the emergence of symptoms of metabolic syndrome, which include weight gain, high cholesterol, and elevated blood pressure. To reiterate, the bacteria present are causing inflammation which can lead to insulin resistance which in turn makes the mice eat more. This study on mice shows the important of applying this concept to humans. Studies on humans can indicate that the tendency to eat is driven by the change in bacteria in intestines; people consume more since their appetite increase to the the low grade inflammation produced. Future studies like this will reveal how pathogens like bacteria can cause disease.
Another study also found a link between gut bacteria and obesity. Researchers at Cedars-Sinai Medical Center indicate that people with high levels of both hydrogen and methane in their breath are more likely to have a higher BMI and higher proportion of body fat. This was the first large-scale study to look at the correlation between gas production and body weight. Tests done on 792 patients indicated that there were four main patterns: normal breath, breath containing higher levels of methane, higher levels of hydrogen, or higher levels of both gases. The gut bacterium, Methanobrevibacter smithii, is responsible for most of the methane produced in the human gut. M. smithii produces methane by scavenging hydrogen from other microorganisms. Abnormally high levels of M. smithii can alter the energy balance leading people to put on more weight. This study creates a direct link between gut bacteria and weight gain by looking at gas production created by gut bacteria.
Researchers have started to look out how bacteria can be used to treat obesity without surgery. Studies on mice have shown that gastric bypass surgery can lead to different types of bacteria to colonize in the gut. Transfer of these colonized bacteria present after surgery into healthy mice led to these healthy mice to rapidly lose weight without surgery. This study highlights the fact that bacteria microbiota is much different in the stomach and intestines of obese people compared with those of people of normal weight. If gastric bypass surgery occurs, the gut microbiota will change.
A study was designed by looking at three groups of obese mice on high calorie diets: 1) group of mice that underwent gastric bypass surgery, 2) group of mice that underwent a fake surgery and the higher calorie diet continued, and 3) group of mice that was give same fake operation but then fed low calorie diet to promote weight loss. The results indicate that only the group that underwent gastric bypass surgery had any change in the micro-organisms present in the stomach and intestines. These microbiota were then transferred to germ-free mice. The group that received the bacteria from bypass mice had the most significant amount of weight loss while the other two groups had no change in weight. This study shows how certain gut bacteria populations can influence weight loss. Future studies will begin to look at the mechanisms by which a microbial population changed due to gastric bypass and its effects on environment.
References
1.Arumugam, M. et al. Nature Advance online publication doi:10.1038/nature09944 (2011)
2.Benson A et al. (2010) Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. October 11, 2010, doi:10.1073/pnas.1007028107 PNAS November 2, 2010 vol. 107no. 44 18933-18938
3.Chierico et al. (2012)Early-life gut microbiota under physiological and pathological conditions: The central role of combined meta-omics-based approaches. Journal of Proteomics Volume 75, Issue 15, 3 August 2012, Pages 4580–4587
4.CDC. Obesity and Overweight. 2013.
5.Sekirov I, Russell S, Caetano L, Antunes M, Finlay B. (2010) Gut Microbiota in Health and Disease. doi: 10. 1152/ physrev. 00045. 2009 Physiol Rev July 1, 2010 vol. 90no. 3 859-904
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.