Listeria monocytogenes Preservative Resistance
Section
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduction
By Iris Pardue
Listeria monocytogenes is a facultatively anaerobic Gram-positive food pathogen of the phylum Bacillota capable of surviving and growing at low temperatures. As a result, it is responsible for infections in deli meats, cheeses, and other refrigerated products.[1] Outbreaks are relatively frequent, and serious cases have an especially high fatality rate of 15.26% over recorded cases in the US in the last decade. Unsurprisingly, much research has been conducted into control of Listeria as a result. Many different methods are used by food supply companies to inhibit bacterial growth, and chief among these methods are chemical preservatives. There are several different types of preservatives commonly used[2], and many have different mechanisms of action that will be discussed later. Having a diverse portfolio of antimicrobial preservatives available is especially important given the ability of pathogens to quickly evolve resistance to common mechanisms. This article explores the variety of commonly used preservatives relevant to controlling listeriosis along with the susceptibilities and resistances possessed by Listeria to each.
Cold Resistance
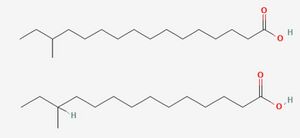
To fully understand the resistance of Listeria to preservatives, it is important to first consider its most importance resistance to cold. Listeria is a psychrophile, capable of growing at freezing temperatures as well as at human body temperatures. [3] Numerous mechanisms exist to aid resistance to cold, but chief among them is adaptations in the content of the phospholipid membrane. This adaptation is mainly achieved via the use of the two primary fatty acids in the lipid membrane, anteiso-17-0 and anteiso-15-0.[4] The proportion between these two fatty acids is modulated in response to low temperatures until the more flexible anteiso-15-0 dominates, reaching 80% of the total fatty acid profile. This adaptation allows it to maintain the crucial "liquid-crystal" state, which is necessary for cell function[5] by lowering the melting point of the membrane. However, the focus on lipids alone can paint an oversimplified picture of the cell membrane's temperature stability. In many species, cholesterol acts as a cellular antifreeze to aid membrane stability[6], and Listeria employs a similar mechanism using isoprenoid quinones[4]. Menaquinone-7 (MK-7), also known as vitamin K2, is composed of a napthaquinone ring fixed to a long chain of 7 isoprene units, which allows it to integrate into the membrane, a structure shared with the quinones used in electron transport. Flegler et al. found that strains containing higher levels of these molecules in their membranes better survived temperature stresses and did not adapt their fatty acid composition to the same degree as other strains. However, product inhibition of the shikimate pathway responsible for synthesis of aromatic amino acids that are processed into quinones did reduce the ability of high MK-7 strains to adapt to the lower temperatures, providing a possible target for future preservatives.
Acid Preservatives
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Ranjan K. Mohapatra, Snehasish Mishra, Lawrence Sena Tuglo, Ashish K. Sarangi, Venkataramana Kandi, Amani Ahmed AL Ibrahim, Hussain A. Alsaif, Ali A. Rabaan, Md. Kudrat-E Zahan. Recurring food source-based Listeria outbreaks in the United States: An unsolved puzzle of concern? Health Science Reports 2024 7:2.
- ↑ Elisabeth Anderson. Preservatives – Keeping our foods safe & fresh.
- ↑ Jones GS, D'Orazio SEF. Listeria monocytogenes: cultivation and laboratory maintenance. Curr Protoc Microbiol. 2013 Nov 5;31:9B.2.1-9B.2.7.
- ↑ 4.0 4.1 Alexander Flegler, Vanessa Kombeitz & André Lipski. Menaquinone-mediated regulation of membrane fluidity is relevant for fitness of Listeria monocytogenes. Arch Microbiol 203, 3353–3360.
- ↑ Richard Templer and John Seddon. The World of Liquid Crystals. NewScientist 18 May 1991.
- ↑ Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720-31.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024